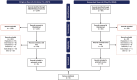
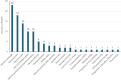
Cost-effectiveness modeling for gender-neutral human papillomavirus vaccines: A systematic literature review
- PMID: 40665636
- PMCID: PMC12269698
- DOI: 10.1080/21645515.2025.2516322
Cost-effectiveness modeling for gender-neutral human papillomavirus vaccines: A systematic literature review
Abstract
Gender-neutral vaccination (GNV) of human papillomavirus (HPV) may help reduce the transmission and incidence of HPV-related diseases. However, approximately 40 countries have implemented HPV GNV schedules. We systematically evaluated HPV GNV cost-effectiveness models from January 2008 to May 2024 using MEDLINE, Embase, and Cochrane to identify key drivers of cost-effectiveness results. Fifty-three publications were included, primarily from high-income countries. Vaccine coverage, price, protection duration, and discount rates impacted cost-effectiveness, with lower prices and protection against HPV-related diseases resulting in cost-effective results. Results in models that included adults (≥18 years) were mixed and dependent on price, inclusion of non-cervical HPV-related diseases, and age groups considered. We conclude that HPV GNV can be a cost-effective strategy for preventing HPV-related diseases. However, its cost-effectiveness is highly dependent on vaccine coverage, price, and inclusion of non-cervical HPV-related diseases in models. Further economic evaluations of HPV GNV in low- and middle-income countries are recommended.
Keywords: Human papillomavirus; cervical cancer; cost-effectiveness; economic evaluation; intervention; screening; vaccination.
Conflict of interest statement
Marisa Felsher, Marcie Fisher-Borne, Tufail Malik, Wei Wang, and Cody Palmer are employees of Merck & Co., Inc. Nita Santpurkar, Stephan Martin, and Omer Zaidi are employees of OPEN Health, which received funding from Merck & Co., Inc. in connection with this publication(s).
Figures
Similar articles
-
Public health impact and cost-effectiveness of implementing gender-neutral vaccination with a 9-valent HPV vaccine in Japan: a modeling study.J Med Econ. 2025 Dec;28(1):974-985. doi: 10.1080/13696998.2025.2520703. Epub 2025 Jun 20. J Med Econ. 2025. PMID: 40528568
-
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.Cochrane Database Syst Rev. 2018 May 9;5(5):CD009069. doi: 10.1002/14651858.CD009069.pub3. Cochrane Database Syst Rev. 2018. PMID: 29740819 Free PMC article.
-
Cost-Effectiveness of 9-Valent HPV Vaccination for Patients Treated for High-Grade Cervical Intraepithelial Neoplasia in the UK.JAMA Netw Open. 2024 Oct 1;7(10):e2437703. doi: 10.1001/jamanetworkopen.2024.37703. JAMA Netw Open. 2024. PMID: 39365579 Free PMC article.
-
Development of a quality framework for models of cervical screening and its application to evaluations of the cost-effectiveness of HPV vaccination in developed countries.Vaccine. 2015 Jan 1;33(1):34-51. doi: 10.1016/j.vaccine.2014.08.048. Epub 2014 Aug 27. Vaccine. 2015. PMID: 25171843
-
Extending the Human Papillomavirus Vaccination Programme to Include Males in High-Income Countries: A Systematic Review of the Cost-Effectiveness Studies.Clin Drug Investig. 2015 Aug;35(8):471-85. doi: 10.1007/s40261-015-0308-4. Clin Drug Investig. 2015. PMID: 26187455
References
-
- World Health Organization . Immunization, vaccines and biologicals: human papillomavirus vaccines (HPV). [accessed 2024 Nov 23]. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases...).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical