Epidemiology of Antiretroviral Therapy Related Adverse Drug Reactions and its Predictors Among Patients with Human Immunodeficiency Virus/AIDS in Ethiopia: A Systematic Review and Meta-analysis
- PMID: 40665675
- PMCID: PMC12268142
- DOI: 10.1177/23259582251358929
Epidemiology of Antiretroviral Therapy Related Adverse Drug Reactions and its Predictors Among Patients with Human Immunodeficiency Virus/AIDS in Ethiopia: A Systematic Review and Meta-analysis
Abstract
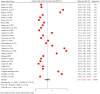
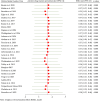
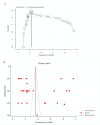
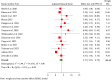
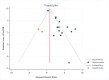
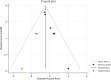
ObjectiveAdverse drug reactions (ADRs) induce iatrogenic harm in antiretroviral therapy (ART) care continuum. However, there is a dearth of concrete evidence in a resource-limited setting. Thus, this study was designed to consolidate existing knowledge, thereby informing policy and clinical care to improve patient safety.DesignSystematic review and meta-analysis.Data sourcesPubMed, CINAHL, Web of Science, and EMBASE databases were searched.Eligibility criteriaEmploying the condition, context, and population framework, observational primary studies were included.Data extraction and synthesisIndependent reviewers undertook data extraction and synthesis. This meta-analysis employed the random-effects restricted maximum likelihood (REML) method, with its protocol preregistered on the International Register of Systematic Reviews (CRD42024546390).ResultsThe pooled prevalence of ADRs was 36.7% [95% CI: 26.6-46.9, I2 = 99.64%].ConclusionAltogether, this study revealed that ART-related ADRs in Ethiopia was 36.7%, underscoring rigorous monitoring. Giving special emphasis to patients with female gender, advanced disease, comorbidities, malnutrition, TB treatment, and poor adherence is a prudent decision.
Keywords: adverse drug reaction; antiretroviral therapy; highly active antiretroviral therapy; human immunodeficiency virus/AIDS; patient safety.
Plain language summary
Epidemiology of ART-related Adverse Drug Reactions among Patients with HIV/AIDS in Ethiopia: A Systematic Review and Meta-analysisMedicines are crucial in the arena of the healthcare system. Despite the undeniable relevance of medicines, a substantial challenge is the potential for harmful drug reactions when used. Antiretroviral therapy (ART) is the application of antiretroviral medicines to combat human immunodeficiency virus (HIV). While it is pivotal in treating diseases, it is plugged with adverse drug reactions (ADRs). ADRs are harmful effects that can occur when people take medications. These undesirable drug effects are quite common and especially costly in the treatment of HIV. In countries with limited healthcare resources, there is limited information about how common these harmful effects are. This study aimed to bring together the available evidence about the rate of ADRs related to ART among people living with HIV in Ethiopia. In this study, we reviewed the results of 29 studies involving 19,003 people living with HIV. We found that about 4 in every 10 patients (36.7%) experienced ADRs from their treatment. Our analysis also showed that certain groups, including women, those with lower education levels, advanced HIV disease, other chronic health problems, weaker immune systems (low CD4 counts), opportunistic infections, malnutrition, and those taking medications containing zidovudine or nevirapine, were more likely to face these ADRs. Patients receiving tuberculosis treatment at the same time or struggling with medication adherence were also at higher risk. Based on these findings, special attention should be given to monitoring these vulnerable groups to catch and manage ADRs early.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Data Availability StatementWe have provided all crude data that was utilized in this investigation as Supplemental materials.
Figures
Similar articles
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article.
-
Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure.Cochrane Database Syst Rev. 2007 Jan 24;2007(1):CD002835. doi: 10.1002/14651858.CD002835.pub3. Cochrane Database Syst Rev. 2007. PMID: 17253483 Free PMC article.
-
Health related quality of life and its association with social support among people living with HIV/AIDS receiving antiretroviral therapy in Ethiopia: a systematic review and meta-analysis.Health Qual Life Outcomes. 2022 May 8;20(1):77. doi: 10.1186/s12955-022-01985-z. Health Qual Life Outcomes. 2022. PMID: 35527300 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Co-formulated abacavir-lamivudine-zidovudine for initial treatment of HIV infection and AIDS.Cochrane Database Syst Rev. 2013 Mar 28;2013(3):CD005481. doi: 10.1002/14651858.CD005481.pub3. Cochrane Database Syst Rev. 2013. PMID: 23543540 Free PMC article.
References
-
- Brunton LL, Knollman BC. Chemotherapy of infectious diseases. In: MacDougall C. (ed) Goodman & Gilman's the pharmacological basis of therapeutics. 14th ed. McGraw Hill LLC; 2023:1227–1334.
-
- World Health Organization Technical Report Services. International drug monitoring: the role of national centres. Report of a WHO meeting. Vol 4981972:1-25. - PubMed
-
- Moirana EL. The Prevalence of antiretroviral-therapy-related adverse reactions, hospitalisation, and mortality among people living with HIV in Africa-A systematic review and Meta-Analysis. Faculty of Health Sciences, Department of Public Health and Family Medicine, University of Cape Town; 2022.
-
- World Health Organization. Medication without harm-WHO global patient safety challenge. World Health Organization; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

