Secretion of transthyretin: molecular mechanisms dependent on the endoplasmic reticulum
- PMID: 40666113
- PMCID: PMC12259610
- DOI: 10.3389/fphys.2025.1623185
Secretion of transthyretin: molecular mechanisms dependent on the endoplasmic reticulum
Abstract
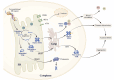
Hereditary transthyretin amyloidosis (ATTRv) results from genetic mutations that destabilize transthyretin (TTR), leading to the formation of extracellular aggregates and amyloid fibrils. A common pathological feature of ATTRv is the capacity of TTR variants to evade endoplasmic reticulum quality control (ERQC) and be secreted, underscoring the critical role of ER regulation in disease pathogenesis. Notably, the TTR Gly83Arg mutation causes familial vitreous amyloidosis, a subtype distinguished by abnormal TTR deposition in the ocular vitreous cavity. Current therapies for ATTRv are ineffective in crossing the blood-retinal barrier or in halting the progression of ocular amyloidosis. This review summarizes the molecular mechanisms of ER-regulated TTR secretion and explores potential causes of ocular amyloid deposition, aiming to provide mechanistic insights into familial vitreous amyloidosis.
Keywords: endoplasmic reticulum; endoplasmic reticulum quality control; transthyretin; unfolded protein response; vitreous amyloidosis.
Copyright © 2025 Meng and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adams D., Tournev I. L., Taylor M. S., Coelho T., Planté-Bordeneuve V., Berk J. L., et al. (2023). Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid 30 (1), 1–9. 10.1080/13506129.2022.2091985 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

