This is a preprint.
Wearable Myoelectric Interface for Neurorehabilitation (MINT) to Recover Arm Function: a Randomized Controlled Trial
- PMID: 40666333
- PMCID: PMC12262789
- DOI: 10.1101/2025.06.24.25330240
Wearable Myoelectric Interface for Neurorehabilitation (MINT) to Recover Arm Function: a Randomized Controlled Trial
Abstract
Background: Abnormal muscle co-activation contributes to arm impairment after stroke. This single-blind, randomized, sham-controlled trial evaluated the feasibility and efficacy of home-based, personalized myoelectric interface for neurorehabilitation (MINT) conditioning to reduce abnormal co-activation and enhance arm function and determine the optimal number of abnormally co-activating muscles to target during training.
Methods: Moderately to severely impaired chronic stroke survivors were randomized to one of three MINT groups (who played customized games requiring independent activation of 2 or 3 abnormally co-activating muscles) or a sham control group (played using one muscle). All groups trained 90 minutes/day, 5 days/week at home and 1 day/week in lab, for 6 weeks, and changed trained muscle sets every 2-3 weeks. The primary outcome was the Wolf Motor Function Test (WMFT) at 6 weeks.
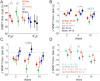
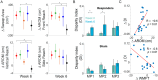
Results: Fifty-nine participants completed the training. Participants performed 315 ± 85 (mean ± SD) repetitions daily. At week 6, participants in all MINT groups combined improved by 4 s on WMFT (p=0.0008), exceeding the minimal clinically important difference (1.5 s). Participants who trained 3 muscles simultaneously improved by 6.8 s (p=0.001), while the 2-muscle and sham groups did not change significantly. In per-protocol analysis, the 3-muscle group, but not 2-muscle groups, improved significantly more than sham (p=0.046), though not in intention-to-treat analysis. All MINT groups continued improving at 4 weeks post-training. Importantly, severely impaired participants in combined MINT groups improved more than those in sham (p=0.02). Importantly, combined MINT groups also improved their reaching range of motion significantly more than sham. Co-activation decreased by 76% in MINT groups during training. Notably, reduction in co-activation during reaching correlated significantly with improved arm function and range of motion. Other secondary outcomes did not show clinically important improvement. Stroke involving the posterior limb of the internal capsule negatively predicted response to MINT.
Conclusions: Home-based MINT conditioning, especially the 3-muscle variant, is feasible, reduces co-activation, and improves arm movement and function.
Clinical trial registration―: ClinicalTrials.gov (NCT03401762).
Keywords: EMG; arm impairment; gaming; movement; myoelectric; stroke recovery; stroke rehabilitation; wearable.
Conflict of interest statement
Disclosures M.W.S. is a consultant for Iota Biosciences, Inc. The remaining authors declare no commercial relationships or conflicts of interest related to this study.
Figures

References
-
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34(9):2181–2186. - PubMed
-
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann. Neurol. 2008;63(3):272–287. - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
