STING regulates NETs formation by activating GSDMD in influenza viral pneumonia
- PMID: 40666504
- PMCID: PMC12260538
- DOI: 10.3389/fimmu.2025.1598902
STING regulates NETs formation by activating GSDMD in influenza viral pneumonia
Abstract
Background: Viral pneumonia is the most common and lethal pandemic disease, but there are no broad-spectrum antiviral drugs with high genetic barriers to resistance. To elucidate the mechanisms of viral pneumonia progression and potential targets for its treatment.
Methods: Viral pneumonia models were induced by the PR8 virus strain in wild-type (WT) and STING knockout (STING-KO) mice. Series of molecular biology techniques were used to evaluate the severity of pneumonia and cytokine levels.
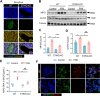
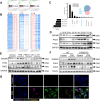
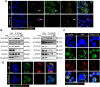
Results: In this study, STING (stimulator of interferon genes) was activated in the lungs of virus-infected mice, leading to cytokine production and amplification of the immune response, thereby causing rapid deterioration of symptoms. Furthermore, excessive activation of innate immune response via STING was prevented by a STING inhibitor (C-176), which significantly reduced viral lung inflammation. The formation of neutrophil extracellular traps (NETs) was similarly suppressed during viral pneumonia treatment with STING inhibitors (C-176), and NETs formation and STING expression were positively correlated, indicating that STING plays an important role in NETs formation. Symptoms of pneumonia in STING-KO mice infected with PR8 were significantly milder than those in WT mice, and NETs were less likely to form in the lung tissue of STING-KO mice. Additionally, transcriptomic analysis revealed that STING-mediated regulation of NETs may be associated with gasdermin D (GSDMD), and immunoprecipitation experiments revealed that STING, GSDMD, and NETs-related proteins interact with each other. Immunofluorescence assays revealed that in neutrophils from WT mice, STING and GSDMD were colocalized on the membrane after viral infection, whereas in neutrophils from STING-KO mice, GSDMD expression was decreased after exposure to the virus.
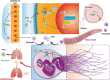
Conclusions: Our study demonstrated that targeted intervention with STING alleviated pneumonia by inhibiting inflammation and NETs formation. The study also revealed that blocking STING could inhibit the activation of GSDMD to inhibit NETs formation, slowing the progression of viral pneumonia.
Keywords: GSDMD; NETs; STING; anti-inflammatory; viral pneumonia.
Copyright © 2025 Huang, Chen, Xing, Wu, Zhu, Jing, Zhou, Wu, Zhang and You.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. (2016) 6:664–79. doi: 10.1158/2159-8290.CD-16-0040 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

