Integration of single-cell RNA and bulk RNA sequencing revealed malignant ductal cell heterogeneity and prognosis signatures in pancreatic cancer
- PMID: 40666516
- PMCID: PMC12259678
- DOI: 10.3389/fimmu.2025.1579184
Integration of single-cell RNA and bulk RNA sequencing revealed malignant ductal cell heterogeneity and prognosis signatures in pancreatic cancer
Abstract
Introduction: Pancreatic cancer is a highly malignant tumor of the digestive system with a dismal prognosis. Despite advances in diagnosis and treatment, overall survival remains extremely low. Early diagnostic markers and an improved understanding of tumor-microenvironment interactions are essential for developing more effective therapies.
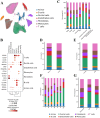
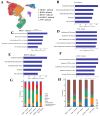
Methods: We analyzed 74 single-cell RNA sequencing (scRNA-seq) samples, performing unsupervised clustering and marker-gene expression profiling to define major cell types. Large-scale chromosomal copy-number variation (CNV) analysis distinguished malignant from non-malignant ductal cells. Non-negative matrix factorization (NMF) identified stage-associated gene modules, which were integrated with TCGA bulk-RNA data and machine-learning feature selection to pinpoint candidate prognostic genes. Two independent cohorts were used for validation. Regulatory network inference (pySCENIC) and ligand-receptor interaction analysis (CellPhoneDB) explored cross-talk between malignant cells and macrophages. Finally, in vitro knockdown of CTSV assessed its functional role in pancreatic cancer (PAC) cell proliferation and migration.
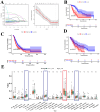
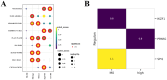
Results: Three prognosis-related genes-ANLN, NT5E, and CTSV-were selected based on their strong association with clinical stage and validated in external datasets. High expression of these genes correlated with poorer overall survival and an increased infiltration of M0 macrophages. CellPhoneDB predicted significant interactions between high-expression malignant ductal cells and M0 macrophages via CXCL14-CXCR4 and IL1RAP-PTPRF axes, with SPI1 identified as an upstream regulator of IL1RAP. In vitro CTSV knockdown significantly inhibited PAC cell proliferation and migration.
Discussion: Our integrative single-cell and bulk-RNA workflow identifies ANLN, NT5E, and CTSV as novel prognostic biomarkers in pancreatic cancer and highlights a pro-tumorigenic interaction between malignant ductal cells and macrophages. Targeting CTSV or disrupting CXCL14-CXCR4 and IL1RAP-PTPRF signaling may offer new therapeutic avenues for PAC.
Keywords: CTSV; CXCL14-CXCR4; macrophages; pancreatic cancer; tumor microenvironment.
Copyright © 2025 Du, Si, Si, Song and Si.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A Novel 14-Gene Panel Associated With Efferocytosis for Predicting Pancreatic Cancer Prognosis Through Bulk and Single-Cell Databases.Front Biosci (Landmark Ed). 2025 Jul 30;30(7):40818. doi: 10.31083/FBL40818. Front Biosci (Landmark Ed). 2025. PMID: 40765357
-
Combined bulk and single-cell transcriptomic analysis reveals cell-type-specific inflammatory crosstalk in pancreatic cancer.Clin Exp Med. 2025 Jul 25;25(1):263. doi: 10.1007/s10238-025-01815-8. Clin Exp Med. 2025. PMID: 40711603 Free PMC article.
-
PLAGL2 as a prognostic biomarker and an EMT-promoting factor in PDAC.Sci Rep. 2025 Jul 14;15(1):25425. doi: 10.1038/s41598-025-09591-x. Sci Rep. 2025. PMID: 40659704 Free PMC article.
-
Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12084-92. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722393 Free PMC article.
-
Roles of lncRNAs in pancreatic ductal adenocarcinoma: Diagnosis, treatment, and the development of drug resistance.Hepatobiliary Pancreat Dis Int. 2023 Apr;22(2):128-139. doi: 10.1016/j.hbpd.2022.12.002. Epub 2022 Dec 14. Hepatobiliary Pancreat Dis Int. 2023. PMID: 36543619
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

