CXCR5 engineered human and murine Tregs for targeted suppression in secondary and tertiary lymphoid organs
- PMID: 40666520
- PMCID: PMC12261343
- DOI: 10.3389/fimmu.2025.1513009
CXCR5 engineered human and murine Tregs for targeted suppression in secondary and tertiary lymphoid organs
Abstract
Introduction: Secondary and tertiary lymphoid structures are a critical target of suppression in many autoimmune disorders, protein replacement therapies, and in transplantation. Although antigen-specific regulatory T cells (Tregs), such as chimeric antigen receptor (CAR) Tregs, generally persist longer and localize to target tissues more effectively than polyclonal Tregs in animal models, their numbers still progressively decline over time. A potential approach to maximize Treg activity in vivo is the expression of chemokine receptors such as CXCR5, which would enable localization of a greater number of engineered cells at sites of antigen presentation. Indeed, CXCR5 expression on follicular T helper cells and follicular Tregs enables migration toward lymph nodes, B cell zones, and tertiary lymphoid structures that appear in chronically inflamed non-lymphoid tissues.
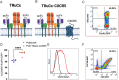
Methods: In this study, we generated human and murine CXCR5 co-expressing engineered receptor Tregs and tested them in preclinical mouse models of allo-immunity and hemophilia A, respectively. Additionally, we engineered a murine CXCR5 co-expressing clotting factor VIII (FVIII) specific T cell receptor fusion construct epsilon (FVIII TRuCe CXCR5) Treg to suppress anti-drug antibody development in a model of FVIII protein replacement therapy for hemophilia A.
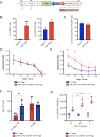
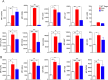
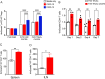
Results: In vitro, anti-HLA-A2 CXCR5+ CAR-Tregs showed enhanced migratory and antigen-specific suppressive capacities compared to untransduced Tregs. When injected into an NSG mouse model of HLA-A2+ pancreatic islet transplantation, anti-HLA-A2 CXCR5+ CAR-Tregs maintained a good safety profile allowing for long-term graft survival in contrast to anti-HLA-A2 CXCR5+ conventional CAR-T (Tconv) cells that eliminated the graft. Similarly, FVIII TRuCe CXCR5 Treg demonstrated increased in vivo persistence and suppressive capacity in a murine model of hemophilia A.
Discussion: Collectively, our findings indicate that CXCR5 co-expression is safe and enhances in vivo localization and persistence in target tissues. This strategy can potentially promote targeted tolerance without the risk of off-target effects in multiple disease models.
Keywords: C-X-C chemokine receptor type 5 (CXCR5); TCR fusion construct epsilon (TRuCε); chimeric antigen receptor (CAR); hemophilia; pancreatic islet transplantation; regulatory T cells (Tregs); type 1 diabetes (T1D).
Copyright © 2025 Doglio, Rana, Stucchi, Melero, Ugolini, Jofra, Toma, Bercher-Brayer, Carulli, Kumar, Monti, Martini, Thirumurugan, Biswas, Bonini and Fousteri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
CD39 delineates chimeric antigen receptor regulatory T cell subsets with distinct cytotoxic & regulatory functions against human islets.Front Immunol. 2024 Jun 28;15:1415102. doi: 10.3389/fimmu.2024.1415102. eCollection 2024. Front Immunol. 2024. PMID: 39007132 Free PMC article.
-
CXCR5-targeted chimeric antigen receptor T regulatory cells for the selective inhibition of follicular helper T cell and B cell interaction.Cytotherapy. 2025 May;27(5):571-580. doi: 10.1016/j.jcyt.2024.12.015. Epub 2025 Jan 6. Cytotherapy. 2025. PMID: 40126458
-
Enhanced homing and efficacy of HER2-CAR T cells via CXCR5/CCR6 co-expression for HER2-positive NSCLC.J Transl Med. 2025 Aug 5;23(1):863. doi: 10.1186/s12967-025-06866-9. J Transl Med. 2025. PMID: 40764989 Free PMC article.
-
Chimeric antigen receptor cell therapy: A revolutionary approach transforming cancer treatment to autoimmune disease therapy.Autoimmun Rev. 2025 Aug 29;24(9):103859. doi: 10.1016/j.autrev.2025.103859. Epub 2025 Jun 23. Autoimmun Rev. 2025. PMID: 40562292 Review.
-
Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells.Biomedicines. 2025 Jul 18;13(7):1757. doi: 10.3390/biomedicines13071757. Biomedicines. 2025. PMID: 40722827 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

