Assessment of ESR1, PGR, ERBB2, and MKI67 mRNA in Hormone Receptor-Positive Early Breast Cancer: A Cross-Sectional Study
- PMID: 40666735
- PMCID: PMC12261032
- DOI: 10.1002/hsr2.71062
Assessment of ESR1, PGR, ERBB2, and MKI67 mRNA in Hormone Receptor-Positive Early Breast Cancer: A Cross-Sectional Study
Abstract
Background and aims: Hormone receptors are expressed in 70% of breast cancers and are the major biomarkers for tailoring treatment in early-stage breast cancer. In clinical routine, immunohistochemistry (IHC) is used to assess estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67 protein expression. However, IHC procedure is challenged with pre-analytical and analytical variability. Pathologist interpretation of IHC results can vary, and discordant results between local and reference laboratories have been reported. Using mRNA-based tests may be a more robust, reliable, and standardized method to assess these important breast cancer biomarkers. This study aimed to assess the concordance between real-time PCR and IHC results.
Methods: In this study, we analyzed 178 early-stage hormone receptor-positive breast tumors. IHC for ER, PR, HER2, and Ki67 had been previously performed for the study samples at local laboratories. For samples with HER2 IHC score 2+, Fluorescence In Situ Hybridization was performed. ESR1 (encoding ER), PGR (encoding PR), ERBB2 (encoding HER2), and MKI67 (encoding Ki67) mRNA expression were determined using TaqMan gene expression assays.
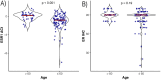
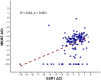
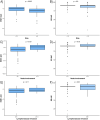
Results: The overall concordance between mRNA expression results and their corresponding IHC markers was 95.9% for ESR1/ER, 79.3% for PGR/PR, and 100% for ERBB2/HER2. There was a moderate correlation between MKi67 mRNA values and Ki67 IHC. ESR1 expression was significantly lower in tumors of younger patients (p < 0.001). No statistically significant correlation between age at cancer diagnosis and ER IHC was identified. Higher ESR1 and MKI67 mRNA expression was associated with worse pathological characteristics.
Conclusions: PCR-based classification of breast tumors in a central laboratory may be used to confirm the available IHC results performed at local laboratories and add valuable information for patient management. mRNA-based biomarkers may be promising for more standardized breast cancer management.
Keywords: HER2 receptor; breast cancer; estrogen receptor; ki67; progesterone receptor.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
ESR1, PGR, ERBB2, and MKi67 mRNA expression in postmenopausal women with hormone receptor-positive early breast cancer: results from ABCSG Trial 6.ESMO Open. 2021 Aug;6(4):100228. doi: 10.1016/j.esmoop.2021.100228. Epub 2021 Aug 7. ESMO Open. 2021. PMID: 34371382 Free PMC article. Clinical Trial.
-
Integrated proteomics and transcriptomics analysis reveals key regulatory genes between ER-positive/PR-positive and ER-positive/PR-negative breast cancer.BMC Cancer. 2025 Jul 1;25(1):1048. doi: 10.1186/s12885-025-14451-y. BMC Cancer. 2025. PMID: 40597935 Free PMC article.
-
The Association between ER, PR, HER2, and ER-/PR+ Expression and Lung Cancer Subsequent in Breast Cancer Patients: A Retrospective Cohort Study Based on SEER Database.Breast J. 2023 Nov 11;2023:7028189. doi: 10.1155/2023/7028189. eCollection 2023. Breast J. 2023. PMID: 38021219 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Cardoso F., Kyriakides S., Ohno S., et al., “Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow‐Up,” Annals of Oncology 30, no. 8 (2019): 1194–1220. - PubMed
-
- Chia S., Norris B., Speers C., et al., “Human Epidermal Growth Factor Receptor 2 Overexpression as a Prognostic Factor in a Large Tissue Microarray Series of Node‐Negative Breast Cancers,” Journal of Clinical Oncology 26, no. 35 (2008): 5697–5704. - PubMed
-
- Harris L. N., Ismaila N., McShane L. M., et al., “Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early‐Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline,” Journal of Clinical Oncology 34, no. 10 (2016): 1134–1150. - PMC - PubMed
-
- Rhodes A., Jasani B., Balaton A. J., and Miller K. D., “Immunohistochemical Demonstration of Oestrogen and Progesterone Receptors: Correlation of Standards Achieved on in House Tumours With That Achieved on External Quality Assessment Material in over 150 Laboratories From 26 Countries,” Journal of Clinical Pathology 53, no. 4 (2000): 292–301. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

