In vitro and in vivo anti- Pseudomonas aeruginosa activity of a scorpion peptide derivative
- PMID: 40666802
- PMCID: PMC12259581
- DOI: 10.3389/fmicb.2025.1622282
In vitro and in vivo anti- Pseudomonas aeruginosa activity of a scorpion peptide derivative
Abstract
Introduction: Pseudomonas aeruginosa is an important opportunistic and foodborne disease-related bacterium, and the increasing antibiotic resistance of the pathogen leads to the urgent exploration of new and effective antibacterial agents. In this study, a scorpion peptide derivative HTP2 was designed.
Methods: The in vitro anti-P. aeruginosa activity was evaluated using a broth microdilution assay. A mouse model of P. aeruginosa skin subcutaneous infection was used to evaluate the in vivo anti-P. aeruginosa activity of HTP2. The antibacterial mechanism and influence on pathogenic factors of P. aeruginosa of HTP2 were also investigated.
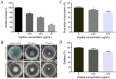
Results: HTP2 could effectively inhibit the growth of P. aeruginosa cells with low hemolytic activity. HTP2 killed P. aeruginosa in a concentration-dependent manner, and could damage the membrane, induce ROS accumulation, and interact with nucleic acids. HTP2 could also inhibit biofilm formation, motility, pyocyanin production, and elastase activity of P. aeruginosa. In the mouse subcutaneous infection model, HTP2 significantly reduced the bacterial load of P. aeruginosa cells and inhibited inflammatory infiltration in the infection area.
Conclusion: HTP2 could effectively kill P. aeruginosa in vitro and in vivo, and had the potential as an anti-P. aeruginosa agent.
Keywords: Pseudomonas aeruginosa; antimicrobial peptide; food contamination; scorpion; skin infection.
Copyright © 2025 Li, Zhang, Liu, Dai, Li, Deng, Wu, Li, Dong, Xin and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Artini M., Imperlini E., Buonocore F., Relucenti M., Porcelli F., Donfrancesco O., et al. (2022). Anti-virulence potential of a Chionodracine-derived peptide against multidrug-resistant Pseudomonas aeruginosa clinical isolates from cystic fibrosis patients. Int. J. Mol. Sci. 23:13494. doi: 10.3390/ijms232113494, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

