This is a preprint.
Evidence that Entomophthora muscae controls the timing of host death via its own circadian clock
- PMID: 40666906
- PMCID: PMC12262267
- DOI: 10.1101/2025.06.18.660419
Evidence that Entomophthora muscae controls the timing of host death via its own circadian clock
Abstract
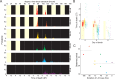
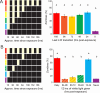
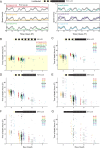
Timing in the natural world is a matter of life or death, consequently, nearly all life on Earth has evolved internal circadian clocks. Many behavior-manipulating parasites exhibit striking daily timing, but whether this is clock-driven has remained unclear. Here, we leveraged the laboratory-tractable zombie fruit fly model, Drosophila melanogaster infected by the behavior manipulating fungus Entomophthora muscae, to tackle this long standing mystery. Using an automated behavioral paradigm, we found that the timing of death of wild-type flies continues to occur with ~21 hour periodicity in the absence of environmental cues. Surprisingly, we also discovered that light is required within the first 24 hours of exposure for E. muscae to infect and kill flies. Experiments with circadian and photoreception mutants revealed that death is independent of host genotype, suggesting the fungus-not the host-drives this rhythm. Transcriptomic analysis of in vitro grown fungus revealed that E. muscae maintains rhythmic gene expression independent of the fly host that peaks at sunset and has a free-running period of ~22 hours. Among cycling genes, we identified a transcript encoding a protein with high homology to white collar-1, the blue light sensor and core component of the molecular oscillator in the model ascomycete fungus Neurospora crassa. Altogether, our findings suggest that E. muscae has an endogenous circadian clock that it uses to control the timing of host death. This study provides evidence that a fungal clock can influence fly outcomes, pointing to a new mechanism by which parasites temporally coordinate host manipulation.
Keywords: Drosophila melanogaster; Entomophthora muscae; behavior manipulation; circadian clock.
Figures


References
-
- Allada R, White NE, So WV, Hall JC, Rosbash M. 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93:791–804. - PubMed
-
- Aschoff J. 1979. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 49:225–249. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
