This is a preprint.
Paclitaxel-induced mitotic arrest results in a convergence of apoptotic dependencies that can be safely exploited by BCL-XL degradation to overcome cancer chemoresistance
- PMID: 40666988
- PMCID: PMC12262480
- DOI: 10.1101/2025.06.24.661170
Paclitaxel-induced mitotic arrest results in a convergence of apoptotic dependencies that can be safely exploited by BCL-XL degradation to overcome cancer chemoresistance
Abstract
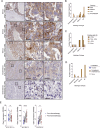
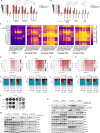
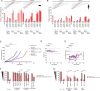
Paclitaxel and other microtubule-targeting agents are cornerstone therapies for diverse cancers, including lung, breast, cervical, pancreatic, and ovarian malignancies. Paclitaxel induces tumor cell apoptosis during mitosis by disrupting microtubule dynamics required for chromosome segregation. However, despite initial responsiveness, many tumors develop resistance, limiting therapeutic durability. Here, we used high-grade serous ovarian carcinoma (HGSOC), the most common and lethal subtype of ovarian cancer, as a model to dissect the mechanisms underlying this resistance. We find that paclitaxel-induced mitotic arrest triggers degradation of the pro-survival protein MCL-1 and upregulation of BCL-XL, followed by inactivating phosphorylation of BCL-XL at Ser62 to promote apoptosis. In resistant cells, this MCL-1 downregulation is insufficient to commit cells to apoptosis but instead results in a transient convergence of apoptotic dependencies by forcing BCL-XL to sequester the pro-apoptotic proteins BIM, BAX, and BAK. During this state, BCL-XL inhibition induces synergistic apoptosis, even in chemoresistant cells. Surprisingly, we also discover that loss of substrate attachment recapitulates this apoptotic convergence both in vitro and in vivo, with HGSOC cells growing in metastasis-promoting malignant ascites displaying heightened apoptotic priming and dependence on BCL-XL relative to solid tumors. In HGSOC xenografts, targeted degradation of BCL-XL using the platelet-sparing proteolysis-targeting chimera (PROTAC) DT2216 matches the efficacy of paclitaxel monotherapy while avoiding the chronic thrombocytopenia induced by BCL-XL inhibitors such as navitoclax (ABT-263). Strikingly, combination therapy leveraging the synergy between paclitaxel and DT2216 leads to complete eradication of HGSOC cell line and patient-derived xenografts. Moreover, DT2216 treatment blunts the rapid apoptotic adaptation caused by other BCL-XL inhibitors, indicating that targeted degradation of pro-survival proteins may yield more durable responses than inhibition alone. These findings uncover a mechanistic framework for safely exploiting the apoptotic dependency convergence caused by mitotic arrest and substrate detachment and support the clinical development of BCL-XL-targeting PROTACs to overcome chemoresistance in ovarian cancer and other solid tumors.
Conflict of interest statement
Declaration of interests: Co-author D.Z. is a co-inventor of DT2216 and a co-founder of and has equity in Dialectic Therapeutics, which develops DT2216 to treat cancer. All other authors declare no competing interests.
Figures




Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Schmid B. C. & Oehler M. K. New perspectives in ovarian cancer treatment. Maturitas 77, 128–36 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
