This is a preprint.
Human Protein Synthesis Requires aminoacyl-tRNA Pivoting During Proofreading
- PMID: 40667029
- PMCID: PMC12262582
- DOI: 10.1101/2025.06.23.661150
Human Protein Synthesis Requires aminoacyl-tRNA Pivoting During Proofreading
Update in
-
Human protein synthesis requires aminoacyl-tRNA pivoting during proofreading.Nat Commun. 2025 Sep 2;16(1):8202. doi: 10.1038/s41467-025-63617-6. Nat Commun. 2025. PMID: 40897704 Free PMC article.
Abstract
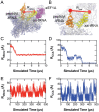
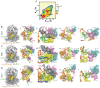
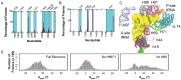
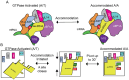
Rigorous studies have characterized the aa-tRNA selection mechanism in bacteria, which is essential for maintaining translational fidelity. Recent investigations have identified critical distinctions in humans, such as the requirement of subunit rolling and a tenfold slower proofreading step. Although these studies captured key intermediates involved in tRNA selection, they did not elucidate the transitions of aa-tRNA between intermediates. Here, we simulated 1,856 aa-tRNA accommodation events into the human ribosomal A site, revealing the requirement of a distinct ~30° pivoting of aa-tRNA about the anticodon stem within the accommodation corridor. This pivoting is crucial for navigating the crowded accommodation corridor, which becomes more constrained due to subunit rolling. Subunit rolling-dependent crowding increases the steric contributions of the accommodation corridor during aa-tRNA accommodation, consistent with the 10-fold reduction in the rate of proofreading. The pivoting of the aa-tRNA enables precise alignment within the accommodation corridor, allowing it to traverse the narrower passage. Furthermore, we found that domain III of eEF1A interacts with the accommodating aa-tRNA through conserved basic residues, providing a steric block to prevent dissociation from the A site. Together, these findings provide a structural framework for understanding the distinctions between bacterial and human aa-tRNA selection and demonstrate that the alignment of the aa-tRNA relative to the ribosomal catalytic sites is a critical determinant of translational fidelity.
Figures



Similar articles
-
Human protein synthesis requires aminoacyl-tRNA pivoting during proofreading.Nat Commun. 2025 Sep 2;16(1):8202. doi: 10.1038/s41467-025-63617-6. Nat Commun. 2025. PMID: 40897704 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Intensive case management for severe mental illness.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD007906. doi: 10.1002/14651858.CD007906.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2017 Jan 06;1:CD007906. doi: 10.1002/14651858.CD007906.pub3. PMID: 20927766 Free PMC article. Updated.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
