This is a preprint.
Synergistic effects of APOE ε4 and Alzheimer's pathology on the neural correlates of episodic remembering in cognitively unimpaired older adults
- PMID: 40667041
- PMCID: PMC12262513
- DOI: 10.1101/2025.06.20.660774
Synergistic effects of APOE ε4 and Alzheimer's pathology on the neural correlates of episodic remembering in cognitively unimpaired older adults
Abstract
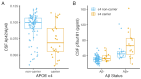
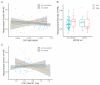
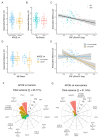
Amyloid-β (Aβ) and tau pathology begin accumulating decades before clinical symptoms and are influenced by APOE ε4, a key genetic risk factor for Alzheimer's disease (AD). Although the presence of Aβ, tau, and APOE ε4 are thought to impact brain function, their effects on the neural correlates of episodic memory retrieval in preclinical AD remains unknown. We investigated this question in 159 cognitively unimpaired older adults (mean age, 68.9±5.8 years; 57% female) in the Stanford Aging and Memory Study. Participants completed an associative memory task concurrent with functional MRI. Aβ was measured using CSF Aβ42/Aβ40 or Florbetaben-PET imaging and tau was measured using CSF pTau181. Hippocampal univariate activity and cortical reinstatement - that is, reinstatement of patterns of neocortical activity that were present during memory encoding - were measured during successful memory retrieval. Analyses revealed that APOE ε4 was independently associated with greater Aβ and tau burden, and that associations of AD biomarkers with brain function and memory were moderated by APOE ε4. Among APOE ε4 non-carriers, Aβ burden was linked to a pattern of hippocampal hyperactivity. Among APOE ε4 carriers, CSF pTau181 was linked to weaker cortical reinstatement during memory retrieval and lower memory performance. Thus, abnormal AD biomarkers and genetic risk synergistically impact neural and behavioral expressions of memory in preclinical AD. These findings highlight the critical role of APOE ε4 in moderating effects of AD pathology on brain function and identify candidate mechanisms that may contribute to increased risk of memory impairment in preclinical AD.
Conflict of interest statement
Conflict of interest statement: The authors declare no competing financial interests.
Figures


Similar articles
-
Alzheimer's disease cerebrospinal fluid biomarker levels and APOE genetic status are associated with hippocampal-cerebellar functional connectivity.Neurobiol Aging. 2025 Jul;151:107-116. doi: 10.1016/j.neurobiolaging.2025.04.005. Epub 2025 Apr 18. Neurobiol Aging. 2025. PMID: 40273528
-
Performance on complex memory tests is associated with β-amyloid in individuals at risk of developing Alzheimer's disease.J Neuropsychol. 2024 Mar;18(1):120-135. doi: 10.1111/jnp.12332. Epub 2023 Jun 29. J Neuropsychol. 2024. PMID: 37382036
-
Influence of APOE ε4 on performance of CSF biomarkers in differentiating clinical Alzheimer's disease.J Prev Alzheimers Dis. 2025 Apr;12(4):100065. doi: 10.1016/j.tjpad.2025.100065. Epub 2025 Jan 17. J Prev Alzheimers Dis. 2025. PMID: 39827005 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
