This is a preprint.
A Human Tumor-Immune Organoid Model of Glioblastoma
- PMID: 40667050
- PMCID: PMC12262450
- DOI: 10.1101/2025.06.16.660009
A Human Tumor-Immune Organoid Model of Glioblastoma
Abstract
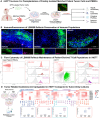
A major obstacle to identifying effective therapies for the aggressive brain tumor glioblastoma is the lack of human-specific, immunocompetent models that reflect the human tumor microenvironment. To address this, we developed the immune-Human Organoid Tumor Transplantation (iHOTT) model. This is an autologous co-culture platform that integrates patient-derived tumor cells and matched peripheral blood mononuclear cells (PBMCs) within human cortical organoids, enabling the study of the patient-specific immune response to the tumor and tumor-immune interactions. This platform preserves tumor and immune populations, immune signaling, and cell-cell interactions observed in patient tumors. Treatment of iHOTT with pembrolizumab, a checkpoint inhibitor, mirrored cell type shifts and cell interactions observed in patients. TCR sequencing further revealed pembrolizumab-driven expansion of stem-like CD4-T-cell clonotypes exhibiting patient-specific repertoires. These findings establish iHOTT as a physiologically relevant platform for exploring autologous tumor-immune interactions and underscore the critical need for antigen-targeted strategies to enhance immunotherapy in glioblastoma.
Conflict of interest statement
DECLARATION OF INTERESTS We (SB, EF, AB) are in the process of filing a provisional patent for the iHOTT system described in this manuscript.
Figures



Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Organoid Models Established from Primary Tumors and Patient-Derived Xenograft Tumors Reflect Platinum Sensitivity of Ovarian Cancer Patients.bioRxiv [Preprint]. 2025 May 2:2024.06.28.601283. doi: 10.1101/2024.06.28.601283. bioRxiv. 2025. PMID: 40654830 Free PMC article. Preprint.
-
Sulindac modulates the response of triple negative breast cancer to anti-PD-L1 immunotherapy.bioRxiv [Preprint]. 2025 Jun 17:2025.06.11.659159. doi: 10.1101/2025.06.11.659159. bioRxiv. 2025. PMID: 40666926 Free PMC article. Preprint.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Tumor organoids in immunotherapy: from disease modeling to translational research.J Immunother Cancer. 2025 Jul 15;13(7):e011733. doi: 10.1136/jitc-2025-011733. J Immunother Cancer. 2025. PMID: 40664453 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. Mar 10;352(10):987–96. - PubMed
-
- Yabo YA, Moreno-Sanchez PM, Pires-Afonso Y, Kaoma T, Nosirov B, Scafidi A, et al. Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts. Genome Medicine. 2024. Apr 2;16(1):51. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
