This is a preprint.
Regulation of multiple paralogs of a small subunit ribosomal protein in Francisella tularensis
- PMID: 40667052
- PMCID: PMC12262407
- DOI: 10.1101/2025.06.29.662229
Regulation of multiple paralogs of a small subunit ribosomal protein in Francisella tularensis
Abstract
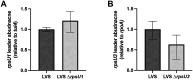
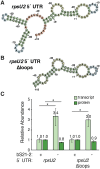
Francisella tularensis is a highly infectious human pathogen that must replicate inside macrophage to cause disease. The ribosomes of F. tularensis can incorporate one of three different paralogs for the small ribosomal subunit protein bS21. One of these paralogs positively impacts translation of key virulence genes and promotes intramacrophage replication. Although ribosomal bS21 content influences F. tularensis virulence, the factors that control bS21 paralog production are not well understood. Here, we reveal that all three bS21 proteins influence the transcript abundance of the paralog important for virulence, bS21-2. In contrast, the other bS21 paralogs (bS21-1 and bS21-3) do not affect their own production. We further determined that the leader sequence of the bS21-2 mRNA is sufficient for bS21-mediated repression of mRNA abundance, suggesting that bS21-2 is autogenously regulated. Yet we determined that the increase in bS21-2-encoding mRNA is not reflected by increased protein production, suggesting that translation of this transcript is controlled by other factors. Finally, we found that bS21-2 exerts at least some of its effects on the bS21-2 transcript by decreasing its stability. Together, our findings suggest that F. tularensis integrates multiple signals into a regulatory network to control the appropriate production of each bS21 paralog, and particularly the paralog important for virulence, bS21-2. This regulatory network in turn may control ribosomal heterogeneity and virulence gene expression.
Conflict of interest statement
Conflicts of Interest The author(s) declare that there are no conflicts of interest.
Figures


References
-
- Ban N, Beckmann R, Cate JH, Dinman JD, Dragon F, Ellis SR, Lafontaine DL, Lindahl L, Liljas A, Lipton JM, McAlear MA, Moore PB, Noller HF, Ortega J, Panse VG, Ramakrishnan V, Spahn CM, Steitz TA, Tchorzewski M, Tollervey D, Warren AJ, Williamson JR, Wilson D, Yonath A, Yusupov M. 2014. A new system for naming ribosomal proteins. Curr Opin Struc Biol 24:165–169. doi: 10.1016/j.sbi.2014.01.002 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
