This is a preprint.
Medial preoptic area FoxO1 controls metabolic adaptation in a sexually dimorphic manner
- PMID: 40667056
- PMCID: PMC12262617
- DOI: 10.1101/2025.06.25.661575
Medial preoptic area FoxO1 controls metabolic adaptation in a sexually dimorphic manner
Abstract
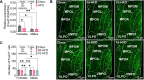
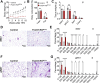
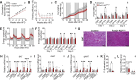
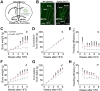
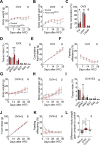
The medial preoptic area (MPOA) of the hypothalamus is essential for metabolic adaptation to environmental challenges, though the molecular mechanisms underlying this process remain poorly understood. Here, we investigate the role of Forkhead transcription factor O1 (FoxO1), a key mediator of stress adaptation, in MPOA-dependent metabolic responses to temperature and nutritional changes. Our findings reveal sex-specific responses to both nutritional and temperature challenges. In female mice, but not males, a high-fat diet (HFD) challenge decreased FoxO1 expression in the MPOA. Specific deletion of FoxO1 in MPOA neurons (FoxO1-KOMPOA) had no effect on body weight under normal chow-fed conditions but protected females from HFD-induced obesity (DIO). These protected females exhibited increased lean mass, decreased fat mass, enhanced thermogenesis, increased energy expenditure, and reduced food intake under HFD conditions. They also showed enhanced cold-induced heat production at 6°C, though this effect vanished at thermoneutrality (30°C). The protection against DIO was abolished by ovariectomy (OVX) and was not restored by 17β-estradiol supplementation, suggesting an estrogen-independent mechanism. Conversely, constitutive activation of FoxO1 in MPOA neurons (FoxO1-CAMPOA) increased DIO susceptibility in both sexes. Together, these findings demonstrate that FoxO1MPOA plays a crucial role in coordinating metabolic adaptation to nutritional and temperature challenges specifically in female mice.
Keywords: FoxO1; MPOA; sex difference.
Figures


References
-
- Gluckman P. D., Beedle A., Buklijas T., Low F. & Hanson M. A. (Oxford University Press, Oxford, 2016).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
