This is a preprint.
CFIm25-Dependent Alternative Polyadenylation in AKT2 mRNA Programs Macrophage Polarization
- PMID: 40667110
- PMCID: PMC12262724
- DOI: 10.1101/2025.06.19.660629
CFIm25-Dependent Alternative Polyadenylation in AKT2 mRNA Programs Macrophage Polarization
Abstract
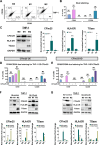
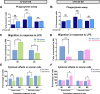
Macrophage polarization is essential for immune responses, tissue homeostasis, and progression of many diseases. It is a tightly regulated process involving an intricate network of signaling pathways and control mechanisms at the level of transcription, alternative mRNA splicing, translation and mRNA stability. However, regulation through alternative mRNA polyadenylation (APA), remains poorly understood. This study explores the function of CFIm25, a key APA regulator, in macrophage polarization. Our findings show that CFIm25 overexpression drives M1 polarization, as evident from increased nitric oxide synthase activity, CD80 expression, and pro-inflammatory cytokine secretion, but dampens the M2 phenotype. Conversely, CFIm25 knockdown suppresses M1 traits and promotes M2 characteristics. Functionally, CFIm25 enhances phagocytosis, migration, and cancer cell inhibition. Mechanistically, CFIm25 favors proximal polyadenylation site usage of AKT2 mRNA, increasing Akt2 protein levels to support M1 polarization. Blocking this site with an antisense oligonucleotide reduces Akt2 expression and M1 traits. These findings establish CFIm25 as a crucial regulator of macrophage identity, offering insights into RNA-based immune regulation and potential therapeutic targets.
Keywords: AKT2; APA; CFIm25; M1/M2 macrophages; Macrophage polarization; NF-κB signaling.
Conflict of interest statement
Declaration of interests No competing interests.
Figures


Similar articles
-
The alternative polyadenylation regulator CFIm25 promotes macrophage differentiation and activates the NF-κB pathway.Cell Commun Signal. 2025 Feb 28;23(1):115. doi: 10.1186/s12964-025-02114-1. Cell Commun Signal. 2025. PMID: 40022203 Free PMC article.
-
[Mechanisms of Neiyiting Decoction in Preventing Postoperative Recurrence of Endometriosis by Inhibiting Macrophage M1 Polarization Through the TREM1/TLR4/NF-κB Signaling Pathway].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Mar 20;56(2):371-381. doi: 10.12182/20250360601. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40599271 Free PMC article. Chinese.
-
Myricetin alleviates DNCB-induced atopic dermatitis by modulating macrophage M1/M2 polarization.Int Immunopharmacol. 2025 Jul 23;163:115212. doi: 10.1016/j.intimp.2025.115212. Online ahead of print. Int Immunopharmacol. 2025. PMID: 40706204
-
Phytochemicals Alleviate Tumorigenesis by Regulation of M1/M2 Polarization: A Systematic Review of the Current Evidence.Phytother Res. 2025 Jul;39(7):3105-3147. doi: 10.1002/ptr.8522. Epub 2025 May 20. Phytother Res. 2025. PMID: 40393795
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
