This is a preprint.
Cholesterol promotes the formation of dimers and oligomers of the receptor tyrosine kinase ROR1
- PMID: 40667148
- PMCID: PMC12262255
- DOI: 10.1101/2025.06.19.660507
Cholesterol promotes the formation of dimers and oligomers of the receptor tyrosine kinase ROR1
Abstract
ROR1 is a member of the receptor tyrosine kinase (RTK) family that plays a crucial role during organogenesis of bone and neural systems by regulating non-canonical Wnt signaling. Misregulation of ROR1 is additionally a causative factor for carcinogenesis in solid and liquid tumors. However, we have a poor understanding of how ROR1 activity is regulated. We employed a recently developed single-molecule method termed SiMPull-POP to study the oligomeric state of ROR1. RTK function is typically triggered by ligand binding, which promotes self-assembly of RTKs to form dimers and in some cases oligomers. However, our data indicate that ROR1 does not follow this paradigm. Instead, ROR1 forms dimers and oligomers in a process that is not affected by the presence of the ROR1 ligand Wnt5a. Additional experiments indicate that the transmembrane domain of ROR1 has a strong tendency to self-assemble, suggesting that this domain modulates ROR1 dimerization. Investigation into a regulatory mechanism for ROR1 self-assembly led to evaluation of the role of the lipid cholesterol, which plays pleiotropic roles in Wnt signaling. Cholesterol was found to promote the assembly of ROR1, and our results point to the transmembrane domain as the region where cholesterol exerts the regulatory effect. Taken together, our results indicate that ROR1 self-assembles in human cells; however, unlike other RTKs, this process is not stabilized by ligand binding but is instead facilitated by membrane cholesterol.
Figures


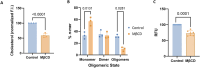
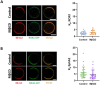
Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Adenovirus Modulates Toll-Like Receptor 4 Signaling by Reprogramming ORP1L-VAP Protein Contacts for Cholesterol Transport from Endosomes to the Endoplasmic Reticulum.J Virol. 2017 Feb 28;91(6):e01904-16. doi: 10.1128/JVI.01904-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077646 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
References
-
- Fukuda T., Chen L., Endo T., Tang L., Lu D., Castro J. E., Widhopf G. F., Rassenti L. Z., Cantwell M. J., Prussak C. E., Carson D. A., and Kipps T. J. (2008) Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proceedings of the National Academy of Sciences 105, 3047–3052 - PMC - PubMed
-
- Billiard J., Way D. S., Seestaller-Wehr L. M., Moran R. A., Mangine A., and Bodine P. V. N. (2005) The Orphan Receptor Tyrosine Kinase Ror2 Modulates Canonical Wnt Signaling in Osteoblastic Cells. Molecular Endocrinology 19, 90–101 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
