This is a preprint.
Comprehensive investigation of SARS-CoV-2 intestinal pathogenesis in Drosophila
- PMID: 40667169
- PMCID: PMC12262708
- DOI: 10.1101/2025.06.25.661044
Comprehensive investigation of SARS-CoV-2 intestinal pathogenesis in Drosophila
Abstract
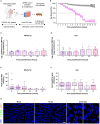
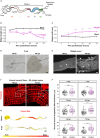
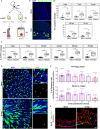
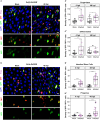
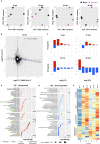
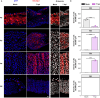
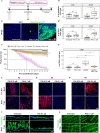
Gastrointestinal (GI) manifestations have been increasingly reported in COVID-19 patients. Here, we use the Drosophila melanogaster midgut model to investigate SARS-CoV-2-induced GI pathogenesis. The fly midgut exhibits susceptibility to orally administered virus, resulting in disrupted epithelial architecture, reduced organ size, and altered visceral muscle dynamics. These effects are accompanied by sustained proliferation of intestinal stem cells alongside decreased replenishment and viability of differentiated cells. Transcriptomic profiling reveals biphasic perturbations in midgut gene expression, particularly in pathways related to lipid metabolism. Intriguingly, SARS-CoV-2 elicits a dichotomous effect on lipid homeostasis, with lipid droplet accumulation in the posterior midgut and depletion in anterior segments. Treatment with Plitidepsin, a COVID-19 drug candidate, mitigates most SARS-CoV-2 pathogenic features in both the Drosophila midgut and human pulmonary cells, while modulating basal lipid droplet homeostasis in uninfected conditions. These findings establish the Drosophila midgut as a potent model for studying SARS-CoV-2 GI pathogenesis and evaluating antiviral compounds.
Keywords: Antivirals; Drosophila; Intestine; Lipid droplets; Plitidepsin; SARS-CoV-2.
Conflict of interest statement
Disclosure and competing interests statement The authors declare no competing interests.
Figures
Similar articles
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Multidisciplinary collaborative guidance on the assessment and treatment of patients with Long COVID: A compendium statement.PM R. 2025 Jun;17(6):684-708. doi: 10.1002/pmrj.13397. Epub 2025 Apr 22. PM R. 2025. PMID: 40261198
References
-
- Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J Virol. 2012;86: 3995–4008. doi: 10.1128/JVI.06540-11 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
