This is a preprint.
Multiple groups of neurons in the superior colliculus convert value signals into saccadic vigor
- PMID: 40667199
- PMCID: PMC12262230
- DOI: 10.1101/2025.06.24.661386
Multiple groups of neurons in the superior colliculus convert value signals into saccadic vigor
Update in
-
Multiple groups of neurons in the superior colliculus convert value signals into saccadic vigor.iScience. 2025 Dec 29;29(2):114563. doi: 10.1016/j.isci.2025.114563. eCollection 2026 Feb 20. iScience. 2025. PMID: 41630918 Free PMC article.
Abstract
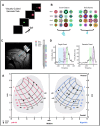
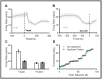
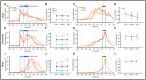
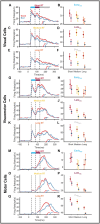
Eye movements directed to high-valued objects in the environment are executed with greater vigor. Superior Colliculus (SC) - a subcortical structure that controls eye movements - contains multiple subtypes of neurons that have distinct functional roles in generating saccades. How does value-related information processed in other parts of the brain affect the responses of these different subtypes of SC neurons to facilitate faster saccades? To test this, we recorded four subtypes of neurons simultaneously while the monkey made saccades to objects they had been extensively trained to associate with large or small rewards (i.e., good or bad). In three subtypes of neurons (visual, visuomotor, and motor), the good objects elicited more spikes than bad objects. More importantly, using a bootstrapping procedure, we identified three separable phases of activity: 1) early visual response (EVIS), 2) late visual response (LVIS), and 3) pre-saccadic (PreSAC) motor response in these neuronal subtypes. In each subtype of neurons, the value of objects (good vs. bad) was positively correlated with the activity in the LVIS and PreSAC phases but not the EVIS phase. These data suggest that the value information from other brain regions modulates the visual (LVIS) and the motor (PreSAC) responses of visual, visuomotor, and motor neurons. This enhanced activation facilitates the faster initiation and execution of the saccade based on the value of each object. In addition, we found a novel class of tonically active neurons that decrease their activity in response to object onset and remain inhibited till the end of the saccade. We suggest that these tonic neurons facilitate the saccade to objects by disinhibiting the interactions between the other three SC neurons.
Keywords: Peak Saccade Velocity; Reaction Time; Reward Modulation; Superior Colliculus; Value-based Behavior.
Conflict of interest statement
Disclosures No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Amador N., Schlag-Rey M., & Schlag J. (2000). Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology, 84(4), 2166–2170. - PubMed
-
- Bahill A. T., Clark M. R., & Stark L. (1975). The main sequence, a tool for studying human eye movements. Mathematical Biosciences, 24(3–4), 191–204.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
