This is a preprint.
Genome editing of phylogenetically distinct bacteria using portable retron-mediated recombineering
- PMID: 40667245
- PMCID: PMC12262390
- DOI: 10.1101/2025.06.16.660010
Genome editing of phylogenetically distinct bacteria using portable retron-mediated recombineering
Abstract
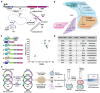
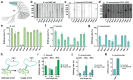
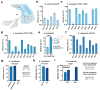
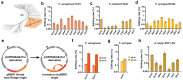
Advanced genome editing technologies have enabled rapid and flexible rewriting of the Escherichia coli genome, benefiting fundamental biology and biomanufacturing. Unfortunately, some of the most useful technologies to advance genome editing in E. coli have not yet been ported into other bacterial species. For instance, the addition of bacterial retrons to the genome editing toolbox has increased the efficiency of recombineering in E. coli by enabling sustained, abundant production of ssDNA recombineering donors by reverse transcription that install flexible, precise edits in the prokaryotic chromosome. To extend the utility of this technology beyond E. coli, we surveyed the portability and versatility of retron-mediated recombineering across three different bacterial phyla (Proteobacteria, Bacillota and Actinomycetota) and a total of 15 different species. We found that retron recombineering is functional in all species tested, reaching editing efficiencies above 20% in six of them, above 40% in three of them, and above 90% in two of them. We also tested the extension of the recombitron architecture optimizations and strain backgrounds in a subset of hosts to additionally increase editing rates. The broad recombitron survey carried out in this study forms the basis for widespread use of retron-derived technologies through the whole Bacteria domain.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures


Similar articles
-
A recombineering-based platform for high-throughput genomic editing in Escherichia coli.Appl Environ Microbiol. 2025 Jul 23;91(7):e0019325. doi: 10.1128/aem.00193-25. Epub 2025 Jun 12. Appl Environ Microbiol. 2025. PMID: 40503884 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Electric fans for reducing adverse health impacts in heatwaves.Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD009888. doi: 10.1002/14651858.CD009888.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786530 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
