This is a preprint.
Integrated Spatial Analysis of Ovarian Precancerous Lesions
- PMID: 40667280
- PMCID: PMC12262305
- DOI: 10.1101/2025.06.24.661327
Integrated Spatial Analysis of Ovarian Precancerous Lesions
Abstract
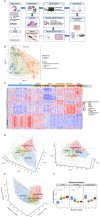
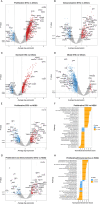
Studying precancerous lesions is essential for improving early detection and prevention, particularly in aggressive cancers such as ovarian carcinoma. Here, we conducted integrated and spatial analyses of transcriptomes, aneuploidy, and clinic-pathological features in 166 ovarian precancerous lesions. Four pre-cancerous subtypes were identified transcriptomically: proliferative, immunoreactive, dormant, and mixed. These subtypes varied in their frequency of germline-BRCA1/2 mutations, aneuploidy, CCNE1/MYC amplification, proliferative activity, immune-regulatory gene expression, and histological features. Notably, the immunoreactive subtype upregulated immune-regulatory genes, exhibited chronic inflammation, and was enriched in cases with germline-BRCA1/2 mutations, deletions of chromosomes 17 (harboring TP53 and BRCA1) and 13 (harboring BRCA2), leading to a double "two-hit" involving TP53 and BRCA1/2. Tumor invasion was associated with the activation of interferon response pathways, epithelial-mesenchymal transition, and extracellular matrix remodeling. In summary, our results elucidate the earliest molecular landscape of ovarian precancerous lesions, serving as the foundation for future risk stratification to identify aggressive pre-cancerous lesions.
Keywords: BRCA1; ovarian cancer; precursor lesions.
Conflict of interest statement
Conflict of interest statement: CD is a consultant and the founder of Belay Diagnostics. He also consults for Exact Sciences. Both companies have licensed technologies from JHU. JHU and CD may be entitled to royalties as part of this arrangement. JHU is aware of the conflict and manages these relationships according to its policy. HC and IS have served as consultants for Roche Diagnostics, Inc.
Figures




References
-
- Shih I-M W.Y., Vang R. (2025). Serous tubal intraepithelial carcinoma—looking into the earliest events of ovarian cancer development. Academia Oncology 2. 10.20935/AcadOnco7620. - DOI
-
- Piek J.M., van Diest P.J., Zweemer R.P., Jansen J.W., Poort-Keesom R.J., Menko F.H., Gille J.J., Jongsma A.P., Pals G., Kenemans P., and Verheijen R.H. (2001). Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195, 451–456. - PubMed
-
- Piek J.M., Verheijen R.H., Kenemans P., Massuger L.F., Bulten H., and van Diest P.J. (2003). BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol 90, 491. S0090825803003652 [pii]. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
