This is a preprint.
Plasma Membrane Damage by Environmental Materials Enhances Cell-Cell Fusion and Impairs Immune Functions of Macrophages
- PMID: 40667368
- PMCID: PMC12262220
- DOI: 10.1101/2025.06.17.660197
Plasma Membrane Damage by Environmental Materials Enhances Cell-Cell Fusion and Impairs Immune Functions of Macrophages
Abstract
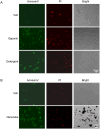
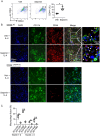
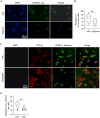
Macrophages are the most abundant phagocytes and play an essential role in host defense. Previous studies have shown that many environmental materials can activate macrophages and trigger inflammatory responses. However, whether these exposures alter macrophage function in host defense remains unclear. This study found that many environmental materials (such as carbon nanotubes, tungsten carbide [WC], and detergents) can damage the plasma membranes of macrophages. This damage leads to decreased reactive oxygen species (ROS) production and phagocytosis but elevated cell-cell fusion. In vivo, airway exposure to laundry detergent impaired the recruitment of macrophages and other myeloid cells to the lung and dramatically dampened protective TH1 and TH17 cell responses, leading to increased susceptibility to Candida infection in mice. Overall, our data indicate that exposure to environmental materials compromises macrophage membrane integrity and impairs host defense. These findings may aid in the development of effective preventive and therapeutic strategies.
Keywords: Environmental Materials; Macrophages; Membrane Damage; Phagocytosis.
Conflict of interest statement
Conflicts of interest. The authors declare that they have no competing interests.
Figures



Similar articles
-
Damage sensing through TLR9 regulates inflammatory and antiviral responses during influenza infection.Mucosal Immunol. 2025 Jun;18(3):537-548. doi: 10.1016/j.mucimm.2025.01.008. Epub 2025 Jan 28. Mucosal Immunol. 2025. PMID: 39884393 Free PMC article.
-
Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages.Elife. 2025 Jun 19;13:RP103714. doi: 10.7554/eLife.103714. Elife. 2025. PMID: 40536105 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
References
-
- Liu L.-Z. et al. Tungsten Carbide-Cobalt Nanoparticles Induce Reactive Oxygen Species, AKT, ERK, AP-1, NF-κB, VEGF, and Angiogenesis. Biol Trace Elem Res 166, 57–65 (2015). - PubMed
-
- Sprince N. L., Chamberlin R. I., Hales C. A., Weber A. L. & Kazemi H. Respiratory Disease in Tungsten Carbide Production Workers. Chest 86, 549–557 (1984). - PubMed
-
- Bolt A. M. & Mann K. K. Tungsten: an Emerging Toxicant, Alone or in Combination. Curr Environ Health Rep 3, 405–415 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
