Transient Dietary Intervention Induces Healthy Adipose Tissue Expansion and Metabolically Healthy Obesity in Mice
- PMID: 40667744
- PMCID: PMC12265394
- DOI: 10.1096/fj.202501121R
Transient Dietary Intervention Induces Healthy Adipose Tissue Expansion and Metabolically Healthy Obesity in Mice
Abstract
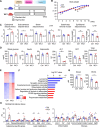
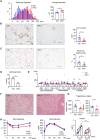
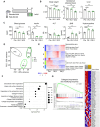
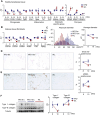
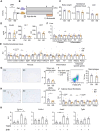
As obesity progresses, dynamic tissue remodeling of adipose tissue occurs over time, that is, adipocyte hypertrophy, chronic inflammation, and interstitial fibrosis. Some obese individuals exhibit healthy adipose tissue expansion, characterized by modest inflammation and fibrosis despite adipocyte hypertrophy, resulting in "Metabolically Healthy Obesity (MHO)". In this study, we investigated the effects of transient weight loss on adipose tissue remodeling during the development of obesity. Male C57BL6/J mice received various types of transient weight loss treatments during diet-induced obesity. A 2-week weight loss intervention during the inflammatory phase promoted healthy adipose tissue expansion, reduced ectopic lipid accumulation, and improved glucose metabolism. In contrast, protocols with shorter duration and delayed intervention, failed to induce MHO. Since serum concentrations of ketone bodies were elevated during weight loss, we examined the effects of hyperketonemia on obesity-induced adipose tissue remodeling. Transient treatment with 1,3-butanediol (BD), which increased serum ketone body concentrations to levels similar to those observed during weight loss, induced healthy adipose tissue expansion and reduced hepatic steatosis even during continuous high-fat diet (HFD) feeding. Ketone bodies effectively suppressed activation of adipose tissue fibroblasts in vivo and in vitro. This study provides evidence that an appropriate dietary intervention can promote healthy adipose tissue expansion in mice, even after the regaining of weight, thereby leading to MHO. As the underlying mechanism, our data revealed a key role for ketone bodies in suppressing activation of adipose tissue fibroblasts. This study paves the way for nutritional approaches to induce MHO.
Keywords: chronic inflammation; fibroblast; fibrosis; ketone body; obesity; weight‐cycling.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Iacobini C., Pugliese G., Blasetti Fantauzzi C., Federici M., and Menini S., “Metabolically Healthy Versus Metabolically Unhealthy Obesity,” Metabolism 92 (2019): 51–60. - PubMed
-
- Suganami T., Tanaka M., and Ogawa Y., “Adipose Tissue Inflammation and Ectopic Lipid Accumulation,” Endocrine Journal 59 (2012): 849–857. - PubMed
MeSH terms
Substances
Grants and funding
- 25KJ0155/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 24K10076/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 24KK0150/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 23K27377/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 23H04776/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 23K16815/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 22K19524/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- JPMJFR230F/MEXT | Japan Science and Technology Agency (JST)
- JP24gm1210009s0106/Japan Agency for Medical Research and Development (AMED)
- JP24fk0210154s0501/Japan Agency for Medical Research and Development (AMED)
- SEI Group CSR Foundation
- Secom Science and Technology Foundation (SSTF)
- Takeda Science Foundation (TSF)
- Food Science Institute Foundation
- Tojuro Iijima Foundation for Food Science and Technology
- TANITA Healthy Weight Community Trust
- Mishima Kaiun Memorial Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials

