Review: Knowledge Gained and Gaps in Understanding in the 25 Years Since Human Metapneumovirus Was First Identified as a Cause of Human Disease
- PMID: 40668102
- PMCID: PMC12265062
- DOI: 10.1093/infdis/jiaf187
Review: Knowledge Gained and Gaps in Understanding in the 25 Years Since Human Metapneumovirus Was First Identified as a Cause of Human Disease
Abstract
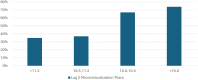
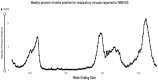
Human metapneumovirus (hMPV) is a nonsegmented, single-stranded, negative-sense RNA virus belonging to the Pneumoviridae family. It was first identified in 2001 in the nasopharyngeal secretions of 28 Dutch children with bronchiolitis collected over a 20-year period. hMPV exhibited paramyxovirus-like morphology with many genetic similarities to respiratory syncytial virus. hMPV has 1 serotype with 2 major subgroups (A and B) and 5 sublineages (A1, A2a, A2b, B1, and B2). In the wake of its discovery, a wealth of observational research has demonstrated global circulation of hMPV causing a wide spectrum of clinical disease. It accounts for 2% to 7% of all symptomatic respiratory infections in children who are universally infected by age 5 years. However, long-lasting immunity to hMPV is incomplete, and reinfections occur throughout life. With increasing age, the impact of hMPV is greater. Adult patients with hMPV infection may develop pneumonia, resulting in hospitalization and severe outcomes, such as intensive care unit admission or mechanical ventilation. Risk factors for severe hMPV are still being defined but include profound immunosuppression (20%), congestive heart failure (25%), and severe chronic obstructive pulmonary disease (20%). In this supplement, several studies from diverse geographic and clinical locations explore the pathogenesis, epidemiology, and clinical profile of hMPV as compared with respiratory syncytial virus and/or influenza and examine the impact of risk factors for severe disease, including age and chronic comorbid conditions. These data are needed to provide the basis for understanding who might benefit from future hMPV vaccines.
Keywords: clinical profile of hMPV infections; epidemiology and global circulation; human metapneumovirus; pathogenesis and immune responses to hMPV; risk for severe disease adults.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. R. B. reports grant funding from Moderna, Cyanvac, Pfizer, Vaccitech, and Merck and serves as a consultant for Novavax, Sanofi, Merck, and GlaxoSmithKline. A. R. B. also received support from AstraZeneca for her efforts to collate this supplemental journal. K. M. E. reports grant funding from the National Institutes of Health and the Centers for Disease Control and Prevention, which ended in December 2022; serves as a consultant to Dynavax and AstraZeneca; and is a member of the data and safety monitoring boards for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and CEPI. K. M. E. also received support from AstraZeneca for her efforts to collate this supplement.
Figures

Similar articles
-
A Comparative Profile of the Burden of Human Metapneumovirus, Respiratory Syncytial Virus, and Influenza in the HIVE Cohort, 2010-2022.J Infect Dis. 2025 Jul 16;232(Supplement_1):S101-S108. doi: 10.1093/infdis/jiaf113. J Infect Dis. 2025. PMID: 40668094 Free PMC article.
-
Respiratory Syncytial Virus and Human Metapneumovirus Respiratory Hospitalizations and Outcomes in Colorado Adults ≥50 Years of Age: 2016-2023.J Infect Dis. 2025 Jul 16;232(Supplement_1):S19-S28. doi: 10.1093/infdis/jiaf266. J Infect Dis. 2025. PMID: 40668095 Free PMC article.
-
The Impact of Chronic Medical Conditions on the Risk of Human Metapneumovirus Hospitalizations in New Zealand Adults, 2012-2015.J Infect Dis. 2025 Jul 16;232(Supplement_1):S59-S68. doi: 10.1093/infdis/jiaf226. J Infect Dis. 2025. PMID: 40668093 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Re-emergence of Human Metapneumovirus: Virus Classification, Characteristics, Mechanisms of Infection, Clinical Features, Diagnosis, Epidemiology, Prevention, and Treatment.Cureus. 2025 Jun 2;17(6):e85259. doi: 10.7759/cureus.85259. eCollection 2025 Jun. Cureus. 2025. PMID: 40612812 Free PMC article. Review.
References
-
- Wolf DG, Zakay-Rones Z, Fadeela A, Greenberg D, Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis 2003; 188:1865–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

