Biofilms and oral health: nanotechnology for biofilm control
- PMID: 40668268
- PMCID: PMC12267812
- DOI: 10.1186/s11671-025-04299-3
Biofilms and oral health: nanotechnology for biofilm control
Abstract
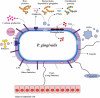
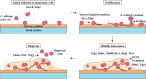
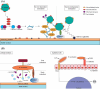
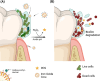
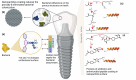
Dental biofilms are complex microbial communities enclosed by a self-produced extracellular matrix, leading to dental caries, periodontitis, and other oral diseases. These biofilms are often resistant to conventional antibiotics and result in persistent infections that negatively impact oral health. Recent advances in nanotechnology have demonstrated nanoparticles as a promising therapeutic alternative for controlling dental biofilms. In addition, such nanoparticles possess unique physicochemical properties such as high surface area-to-volume ratio, enhanced reactivity, and ability to penetrate biofilm structures. Therefore, this review explores the potential of various nanoparticles, such as silver, zinc oxide, and titanium dioxide, in disrupting biofilm formation and removal of pathogenic oral biofilm forming bacteria. Additionally, this review critically examines various strategies for surface functionalization of nanoparticles to enhance their antimicrobial efficacy and biofilm-targeting capabilities. Furthermore, the article also presents various applications of dental materials coated with nanoparticles in preventing biofilm adhesion and growth. In essence, this review article will provide collective information on various approaches in using nanoparticles to reduce the risk of recurrent oral infections and enhance overall dental health.
Keywords: Antimicrobial coating; Biofilm disruption; Dental biofilm; Nanoparticles; Oral health.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interest: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures
Similar articles
-
Recent updates and feasibility of nanodrugs in the prevention and eradication of dental biofilm and its associated pathogens-A review.J Dent. 2024 Apr;143:104888. doi: 10.1016/j.jdent.2024.104888. Epub 2024 Feb 9. J Dent. 2024. PMID: 38342369
-
Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties.Antibiotics (Basel). 2025 May 29;14(6):555. doi: 10.3390/antibiotics14060555. Antibiotics (Basel). 2025. PMID: 40558146 Free PMC article. Review.
-
Effects of Metal and Metal Oxide Nanoparticles against Biofilm-Forming Bacteria: A Systematic Review.J Microbiol Biotechnol. 2024 Sep 28;34(9):1748-1756. doi: 10.4014/jmb.2403.03029. Epub 2024 Jul 15. J Microbiol Biotechnol. 2024. PMID: 39099204 Free PMC article.
-
Deciphering the dynamics of methicillin-resistant Staphylococcus aureus biofilm formation: from molecular signaling to nanotherapeutic advances.Cell Commun Signal. 2024 Mar 22;22(1):188. doi: 10.1186/s12964-024-01511-2. Cell Commun Signal. 2024. PMID: 38519959 Free PMC article. Review.
-
Copper-coated carbon-infiltrated carbon nanotube surfaces effectively inhibit Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation.Appl Environ Microbiol. 2025 Jul 8:e0105325. doi: 10.1128/aem.01053-25. Online ahead of print. Appl Environ Microbiol. 2025. PMID: 40626868
References
-
- Abdulkareem EH, Memarzadeh K, Allaker R, Huang J, Pratten J, Spratt D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J Dent. 2015;43(12):1462–9. - PubMed
-
- Abdullah HN, Mohamad S, WanTaib WR, Jaffar N. Quorum sensing related activity of Aggregatibacter actinomycetemcomitans in periodontal disease: a review. Biomedicine. 2021;41(2):174–80.
Publication types
LinkOut - more resources
Full Text Sources
