Real-world outcomes of first-line treatment with HLX02 versus reference trastuzumab plus pertuzumab in HER2-positive metastatic breast cancer
- PMID: 40668387
- PMCID: PMC12265459
- DOI: 10.1093/oncolo/oyaf183
Real-world outcomes of first-line treatment with HLX02 versus reference trastuzumab plus pertuzumab in HER2-positive metastatic breast cancer
Abstract
Background: HLX02 (Zercepac®) is the first trastuzumab biosimilar manufactured in China. This study presents the first real-world comparison of HLX02 with reference trastuzumab (RTZ) plus pertuzumab and chemotherapy as the first-line treatment for HER2-positive metastatic breast cancer (MBC) patients.
Methods: Medical data of patients with HER2-positive MBC who received HLX02 or RTZ, both combined with pertuzumab and various chemotherapies as the first-line therapy at Beijing Cancer Hospital from January 2019 to August 2023 were reviewed retrospectively. The survival outcomes, efficacy, and adverse events were analyzed.
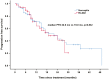
Results: In total, 118 patients were included in this study retrospectively, among whom 66 patients received RTZ and 52 received HLX02. No significant difference was observed in progression-free survival (PFS) between the groups (median PFS: 22.0 months for RTZ vs. 19.0 months for HLX02, P = .832). Additionally, the objective response rate, disease control rate, and safety profiles were similar across both groups. Of all 118 patients, 20 (16.9%) patients experienced progression in the central nervous system (CNS), with a median time to CNS progression of 15.0 months (95% confidence interval, CI, 12.8-17.2).
Conclusion: The real-world data suggested that both HLX02 and RTZ, when combined with pertuzumab and various chemotherapy regimens, offer comparable efficacy and safety as first-line treatments for HER2-positive advanced breast cancer patients in China.
Keywords: HER2 positive; biosimilar; metastatic breast cancer; pertuzumab; trastuzumab.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures
References
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707-712. https://doi.org/ 10.1126/science.2470152 - DOI - PubMed
-
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461-471. https://doi.org/ 10.1016/S1470-2045(13)70130-X - DOI - PMC - PubMed
-
- Xu BH, Li W, Zhang QY, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): final analysis of a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2023;197:503-513. https://doi.org/ 10.1007/s10549-022-06775-1 - DOI - PMC - PubMed
-
- Xu B, Zhang Q, Sun T, et al. ; HLX02-BC01 Investigators. Efficacy, safety, and immunogenicity of HLX02 compared with reference trastuzumab in patients with recurrent or metastatic HER2-positive breast cancer: a randomized phase iii equivalence trial. BioDrugs. 2021;35:337-350. https://doi.org/ 10.1007/s40259-021-00475-w - DOI - PMC - PubMed
-
- Swain SM, Baselga J, Miles D, et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25:1116-1121. https://doi.org/ 10.1093/annonc/mdu133 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous