DNA-incorporated thioguanine to detect potential non-adherence to maintenance therapy in acute lymphoblastic leukemia
- PMID: 40668416
- PMCID: PMC12267380
- DOI: 10.1007/s00280-025-04784-7
DNA-incorporated thioguanine to detect potential non-adherence to maintenance therapy in acute lymphoblastic leukemia
Abstract
Purpose: Adherence to 6-mercaptopurine (6-MP)/methotrexate maintenance treatment for acute lymphoblastic leukemia (ALL) is pivotal to preventing relapse, and the 6-MP metabolite DNA-incorporated thioguanine (DNA-TG) is associated with relapse risk. In the ALLTogether-1 (A2G1) Maintenance sub-study (EU CT nr 2022-501050-11-01), DNA-TG, thioguanine nucleotides (TGN), and methylated mercaptopurine metabolites (MeMP) are analyzed regularly. Upon levels below preset limits (TGN < 50, or MeMP < 200 or < 100 nmol/mmol hemoglobin for thiopurine S-methyltransferase (TPMT) wild type and heterozygous patients, respectively), the treating physician is informed of potential non-adherence. We investigated the feasibility of using DNA-TG as the primary flagging of potential non-adherence.
Methods: We analyzed 6-MP metabolites in 3,074 blood samples from 368 children enrolled in the A2G1 Maintenance sub-study.
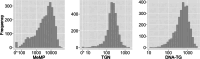
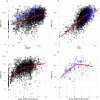
Results: In 6% of samples, TGN (median 212, 95% range 40-642), MeMP (median 4,959, 95% range 135-23,880) or both were below the flagging potential non-adherence limits. DNA-TG was associated with TGN (estimate = 1.72, p < 0.0001), MeMP (estimate = 1.10, p < 0.0001), and prescribed 6-MP dose (estimate = 1.083 and 1.132, p < 0.0001, for TPMT wild type and heterozygous patients) in linear effects models, and the predicted probability of treatment interruption in logistic regression models. DNA-TG was below 200 fmol TG/µg DNA (13th percentile of all measurements, median 569, 95% range 73-1,823) in all samples with both TGN and MeMP below the flagging potential non-adherence limits.
Conclusion: DNA-TG can provide a cost-effective guidance on when to measure TGN and MeMP to determine whether non-adherence should be suspected, which is an additional benefit to monitoring DNA-TG during maintenance therapy.
Keywords: Acute lymphoblastic leukemia; Adherence; DNA thioguanine; Maintenance therapy; Mercaptopurine; Therapeutic drug monitoring.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: A liquid formulation of 6-thioguanine (6TG) is provided by Novalab to the TEAM trial ( www.clinicaltrials.gov no NCT04307576), but the patients receiving 6TG are not included in this study. Ethical approval: This study is part of the A2G Maintenance Therapy sub-study to the ALLTogether1 study (EU CT nr 2022-501050-11-01). Oral and written consent was obtained from parents/caregivers and patients when appropriate. The study was approved on October 28th, 2020, in the European voluntary harmonization process (VHP1557/SA1[VHP2019111/SA1], current amendment accepted November 12th, 2021; VHP1557/SA3[VHP2019111/SA3]), followed by individual approval in participating countries by ethics committees.
Figures
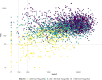
Similar articles
-
Role of TPMT and ITPA variants in mercaptopurine disposition.Cancer Chemother Pharmacol. 2018 Mar;81(3):579-586. doi: 10.1007/s00280-018-3525-8. Epub 2018 Feb 1. Cancer Chemother Pharmacol. 2018. PMID: 29387964
-
Antimetabolite dose intensity and adverse outcomes in children with acute lymphoblastic leukemia: a COG-AALL03N1 report.Blood. 2024 Nov 28;144(22):2327-2335. doi: 10.1182/blood.2024024455. Blood. 2024. PMID: 39190431
-
DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial.Lancet Oncol. 2017 Apr;18(4):515-524. doi: 10.1016/S1470-2045(17)30154-7. Epub 2017 Mar 1. Lancet Oncol. 2017. PMID: 28258828 Clinical Trial.
-
Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2015 Oct 30;2015(10):CD000067. doi: 10.1002/14651858.CD000067.pub3. Cochrane Database Syst Rev. 2015. PMID: 26517527 Free PMC article.
-
Methotrexate for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Aug 11;2015(8):CD007560. doi: 10.1002/14651858.CD007560.pub3. Cochrane Database Syst Rev. 2015. PMID: 26263042 Free PMC article.
References
-
- Toft N, Birgens H, Abrahamsson J et al (2018) Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 32:606–615. 10.1038/leu.2017.265 - PubMed
-
- Nielsen SN, Grell K, Nersting J et al (2017) DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol 18:515–524. 10.1016/S1470-2045(17)30154-7 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

