Pathologic Complete Response and Survival in Rectal Cancer: A Systematic Review and Meta-Analysis
- PMID: 40668580
- PMCID: PMC12268488
- DOI: 10.1001/jamanetworkopen.2025.21197
Pathologic Complete Response and Survival in Rectal Cancer: A Systematic Review and Meta-Analysis
Abstract
Importance: Pathologic complete response (pCR) is increasingly used as a surrogate end point for survival in randomized clinical trials (RCTs) in the field of gastrointestinal oncology. Although this approach has been endorsed by the US Food and Drug Administration, it is still novel and incompletely verified.
Objective: To evaluate the trial-level association between pCR and survival in rectal cancer RCTs examining the effectiveness of neoadjuvant therapy.
Data sources: A meta-analysis was conducted to identify eligible RCTs in PubMed, EMBASE, and Cochrane databases published between database inception to January 3, 2024.
Study selection: RCTs evaluating neoadjuvant therapies in patients with rectal cancer who underwent subsequent surgical resection and reported pCR, overall survival (OS), and disease-free survival (DFS) were included.
Data extraction and synthesis: Two investigators extracted the data, which were verified by a third author. The risk of bias and the certainty of the evidence were evaluated using the Cochrane Risk of Bias Tool, version 2, and the Grading of Recommendations Assessment, Development, and Evaluation tool, respectively. Adjusted odds and hazard ratios with their 95% CIs were extracted. Weighted linear regression was used to assess the correlation between pCR and both OS and DFS.
Main outcomes and measures: Trial-level correlation between pCR and both OS and DFS.
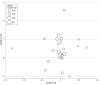
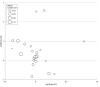
Results: Twenty-five RCTs, including 11 882 patients, met the inclusion criteria. On meta-regression analysis, pCR was not correlated with OS (β = 0.37; 95% CI, -0.98 to 1.71; P = .57). Similarly, pCR was not correlated with DFS (β = -0.84; 95% CI, -2.55 to 0.87; P = .32). Two studies (8%) used in the meta-analysis had high risk of bias. On sensitivity analysis excluding these studies, pCR was still not associated with OS or DFS. Subgroup analysis of RCTs using total neoadjuvant therapies was not possible due to limited sample size.
Conclusions and relevance: In this systematic review and meta-analysis of RCTs comparing neoadjuvant therapies in rectal cancer, there was no trial-level association between pCR and survival. These results suggest that the use of pCR as a surrogate end point for survival should be reexamined and used more cautiously.
Conflict of interest statement
Figures

References
-
- US Food and Drug Administration . Expedited Programs for Serious Conditions—Drugs and Biologics: Guidance for Industry. Accessed May 27, 2025. https://www.fda.gov/media/86377/download
-
- Chitkara A, Rai MP, Thawani R, Chen EY. Recent analysis of frequency of surrogate end points used in oncology clinical trials 2006-2022. J Clin Oncol. 2023;41(16)(suppl). doi: 10.1200/JCO.2023.41.16_suppl.e13658 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

