Tafasitamab plus lenalidomide as salvage therapy in diffuse large B-cell lymphoma: real-world experience from GELTAMO
- PMID: 40668613
- PMCID: PMC12508888
- DOI: 10.1182/bloodadvances.2025016661
Tafasitamab plus lenalidomide as salvage therapy in diffuse large B-cell lymphoma: real-world experience from GELTAMO
Abstract
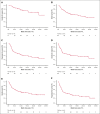
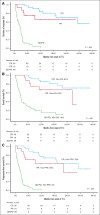
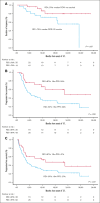
Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) remains challenging to treat, especially in patients ineligible for intensive therapy or chimeric antigen receptor T cells. Tafasitamab plus lenalidomide (T/L) is an effective option based on the phase 2 L-MIND trial findings, although real-world evidence studies have not consistently confirmed these results. We aimed to describe real-world outcomes of R/R DLBCL treated with T/L in Spain. A total of 99 patients received at least 1 dose of tafasitamab (intent-to-treat [ITT] cohort), with 83 completing at least 1 full cycle of T/L (efficacy cohort). Respectively for ITT and efficacy cohorts, at a median follow-up of 19.2 and 21.6 months, the overall response rate was 51% and 61% (complete response [CR], 35% and 42%). Median duration of response was not reached, and patients achieving a CR had excellent outcomes. The median progression-free survival (PFS) was 4.9 and 10.9 months, and overall survival (OS) was 12.2 and 21.8 months, respectively for both ITT and efficacy cohorts. Neither age nor cumulative illness rating score influenced survival. Better PFS was obtained in first/second relapse but only poor Eastern Cooperative Oncology Group performance status 2 to 4, double-hit lymphoma, and those with refractory/progressing disease after the previous therapy, were independently associated with worse PFS. Treatment was generally well tolerated, with manageable toxicity. Relative dose intensity of lenalidomide significantly affected response, PFS, and OS. In summary, T/L is both well tolerated and effective, irrespective of age or comorbidities. Our findings provide valuable insights into the real-world application of T/L and reinforce its role as a key treatment option for patients with R/R DLBCL.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.G. has received research funding and honoraria from Janssen, AbbVie, Roche, AstraZeneca, Incyte, Takeda, Lilly, and BeiGene; M.B.-O. has received research funding and honoraria from Incyte, Roche, AbbVie, and Kite; P.A. has received honoraria from BeiGene, Janssen, Roche, Bristol Myers Squibb, AbbVie, Incyte, Gilead, and AstraZeneca. I.Z. and E.G.-B. disclose honoraria from Incyte. The remaining authors declare no competing financial interests.
Figures

References
-
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. - PMC - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. - PubMed

