Dietary tryptophan augments cancer-associated venous thrombogenicity mitigated by indoleamine 2,3-dioxygenase 1 inhibition
- PMID: 40668615
- PMCID: PMC12508897
- DOI: 10.1182/bloodadvances.2025017079
Dietary tryptophan augments cancer-associated venous thrombogenicity mitigated by indoleamine 2,3-dioxygenase 1 inhibition
Abstract
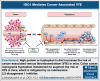
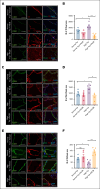
Studies related to cardio-oncology remain a high priority, considering that venous thromboembolism (VTE) in cancer survivors is the second most common cause of death. Although diet-derived metabolites are emerging as contributors to VTE, the influence of specific dietary components, their underlying mechanisms, and means to mitigate cancer-associated VTE remain poorly investigated. This point is important because population studies point to a protein-rich diet associated with VTE. Leveraging a new colon cancer-VTE mouse model, we show that an imbalanced protein-rich diet augments venous thrombogenicity in tumor-bearing mice. Further probing showed that dietary tryptophan induces a procoagulant venous wall, characterized by upregulation of tissue factor, plasminogen activator inhibitor-1, and von Willebrand factor and downregulation of thrombomodulin. Targeted metabolomics of sera from tumor-bearing mice revealed a pattern consistent with increased biogenesis of kynurenine (Kyn) and its suppressed catabolism, despite equal diet consumption in all groups. Kyn levels positively correlated with venous clots. Indoleamine 2,3-dioxygenase 1 (IDO1) is a key rate-limiting enzyme converting tryptophan to Kyn. Sera and the inferior vena cava of tumor-bearing mice showed greater IDO1 activity and protein level, respectively. A specific IDO1 inhibitor reduced serum levels of Kyn, restored the balance of procoagulant and anticoagulant factors in the venous endothelium, and significantly suppressed venous thrombogenicity in tumor-bearing mice. Taken together, our results uncovered a prothrombotic effect of a protein- or tryptophan-rich diet in a syngeneic colon cancer model, which is significantly attenuated by an IDO1 inhibitor.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program . 2020. SEER Cancer Statistics Review (CSR) 1975-2017.
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. 2016;140(Suppl 1):S12–S17. - PubMed
-
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

