IMPACT OF FARICIMAB VERSUS AFLIBERCEPT ON EPIRETINAL MEMBRANE FORMATION OVER 2 YEARS IN PATIENTS WITH DIABETIC MACULAR EDEMA IN THE PHASE 3 YOSEMITE AND RHINE TRIALS
- PMID: 40668667
- PMCID: PMC12548813
- DOI: 10.1097/IAE.0000000000004572
IMPACT OF FARICIMAB VERSUS AFLIBERCEPT ON EPIRETINAL MEMBRANE FORMATION OVER 2 YEARS IN PATIENTS WITH DIABETIC MACULAR EDEMA IN THE PHASE 3 YOSEMITE AND RHINE TRIALS
Abstract
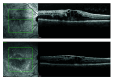
Background/purpose: To assess the effects of faricimab versus aflibercept on epiretinal membrane (ERM) formation in eyes with diabetic macular edema.
Methods: Post hoc analysis of phase 3 YOSEMITE/RHINE trial data in eyes with diabetic macular edema receiving faricimab every 8 weeks (Q8W), faricimab treat-and-extend (T&E; up to Q16W depending on central subfield thickness and best-corrected visual acuity), or aflibercept Q8W for 100 weeks.
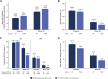
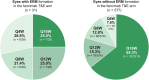
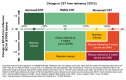
Results: ERMs developed in 3.8% (23/602) of eyes treated with faricimab Q8W, 5.1% (31/608) with faricimab T&E, and 7.6% (45/590) with aflibercept Q8W at 100 weeks. ERMs were less likely with faricimab Q8W versus aflibercept Q8W (odds ratio [OR] 0.48, 95% confidence interval [CI] 0.29-0.81, P = 0.0055). The mean (SD) best-corrected visual acuity at 100 weeks in eyes with and without ERMs were 69.2 (13.6) letters [20/40 Snellen] versus 73.8 (13.1) [20/40 Snellen], respectively; the mean (SD) CSTs were 315.8 (99.2) versus 274.6 (74.1) µ m. Faricimab T&E dosing intervals were extended ≥ Q12W in 79.7% of eyes without ERMs versus 50.0% with ERMs.
Conclusion: Risk of ERMs was 52% lower with faricimab Q8W versus aflibercept Q8W over 100 weeks in eyes with diabetic macular edema, suggesting a potential role for faricimab in reducing pre-retinal fibrotic proliferation. The results may help inform physician/patient decision-making when initiating intravitreal therapy.
Trial registration: NCT03622580 and NCT03622593.
Keywords: angiopoietin-2; diabetic macular edema; epiretinal membrane; faricimab; vascular endothelial growth factor-A.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Ophthalmic Communications Society, Inc.
Conflict of interest statement
G. J. Jaffe: consultant—4D Molecular Therapeutics, Adverum, Annexon, Boehringer Ingelheim, EyePoint Pharmaceuticals, Kriya Therapeutics, Neurotech Pharmaceuticals Inc., Ocular Therapeutix, Regeneron, Ripple Therapeutics, Roche/Genentech, Inc. G. Deák: None. K. Gibson: employee—Roche Products Ltd. R. N. Khurana.: consultant—AbbVie, Bausch + Lomb, Clearside, Genentech, Inc., NGM Bio, Opthea, Regeneron, and RegenxBio; and has received research funding from Annexion, Apellis, Clearside Biomedical, EyePoint Pharmaceuticals, Genentech, Inc., NGM Bio, Opthea, Oxurion, and RegenxBio. E. Nudleman: consultant—Alcon, Allergan/AbbVie, EyeBio, Genentech, Inc. Y. Ogura: consultant—Alcon Japan, Apellis, Astellas, Bayer, Boehringer Ingelheim, Chengdu Kanghong, Chugai Pharmaceutical Co., Ltd., HOYA, Iveric Bio, Kyoto Drug Discovery & Development, Novartis, Senju, and Wakamoto; Lecture fees—Bayer, KOWA, NIKON Healthcare, Novartis, Santen, Sanwa Kagaku, Topcon, and ZEISS; former employee—Genentech, Inc. U. Schmidt-Erfurth: consultant—Apellis, Astellas, Aviceda, Complement Therapeutics, Heidelberg Engineering, Novartis, ONL Therapeutics, RetInSight, Roche, Topcon, Genentech, Kodiak. T. Wang, P. D. Westenskow: employee—F. Hoffmann-La Roche Ltd. D. Wong: consultant—AbbVie, Alcon, Apellis, Bausch Health, Bayer, Biogen, Novartis, Regeneron, Ripple, Roche, ZEISS; Grants; Bayer, Novartis, Roche. G. Yiu: consultant—4D Molecular Therapeutics, AbbVie, Adverum, Alimera, Bausch + Lomb, Boehringer Ingelheim, Clearside Biomedical, Endogena, Genentech, Inc., Gyroscope Therapeutics, Iridex, Janssen, jCyte, Myrobalan, NGM Bio, Novartis, Ocuphire, Ray Therapeutics, Regeneron, RegenxBio, Stealth BioTherapeutics. J. R. Willis: employee—Genentech, Inc.
Figures


References
-
- Akbar Khan I, Mohamed MD, Mann SS, et al. Prevalence of vitreomacular interface abnormalities on spectral domain optical coherence tomography of patients undergoing macular photocoagulation for centre involving diabetic macular oedema. Br J Ophthalmol 2015;99:1078–1081. - PubMed
-
- Bu SC, Kuijer R, Li XR, et al. Idiopathic epiretinal membrane. Retina 2014;34:2317–2335. - PubMed
-
- Hirano M, Morizane Y, Kanzaki Y, et al. En face image-based analysis of retinal traction caused by epiretinal membrane and its relationship with visual functions. Retina 2020;40:1262–1271. - PubMed
-
- Fung AT, Galvin J, Tran T. Epiretinal membrane: a review. Clin Exp Ophthalmol 2021;49:289–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

