Cardiogenic and chronobiological mechanisms in seizure-induced sinus arrhythmias
- PMID: 40668822
- PMCID: PMC12286331
- DOI: 10.1371/journal.pcbi.1013318
Cardiogenic and chronobiological mechanisms in seizure-induced sinus arrhythmias
Abstract
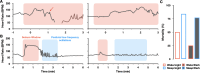
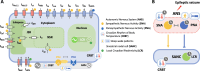
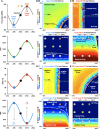
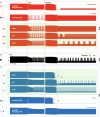
Seizure-induced cardiac arrhythmias, such as ictal (during seizure) or postictal (post-seizure) sinus arrhythmias, are potential triggers for sudden unexpected death in epilepsy. Traditionally, these arrhythmias have been attributed to changes in autonomic balance during ictal or postictal phases, as per the neurogenic mechanism. However, it remains unclear if these arrhythmias may involve intrinsic cardiogenic mechanisms. Furthermore, while circadian and sleep-wake patterns influence both neurogenic and cardiogenic mechanisms, a direct mechanistic link to seizure-induced arrhythmias remains to be established. In this study, we utilized a mathematical model of mouse sinoatrial nodal cell pacemaking and an autonomic clamping protocol, to dissect neurocardiogenic mechanisms in seizure-induced sinus arrhythmias and to test the hypothesis that circadian and sleep-wake rhythms directly modulate cellular susceptibility to these arrhythmias. Our simulations revealed that, in the context of altered autonomic levels associated with seizure progression, diverse seizure-induced sinoatrial nodal cell firing patterns during ictal or postictal phases can be triggered directly by intrinsic cardiac dynamics, without the need for dynamical changes in within-phase autonomic activities. This finding highlights the distinct roles of neurogenic and cardiogenic mechanisms in shaping sinoatrial nodal cell firing patterns, challenging the predominance of the neurogenic mechanism. This neurocardiogenic framework also successfully captures distinct circadian and vigilance state patterns of seizure-induced arrhythmias. Specifically, while daytime sleep predisposed sinoatrial nodal cells to postictal sinus arrhythmias, nighttime wakefulness promotes ictal sinus arrhythmias. However, these circadian patterns can be disrupted when sleep-wake cycles are decoupled from circadian rhythms, supporting the hypothesis that sleep-wake patterns can directly be a key determinant of seizure-induced sinus arrhythmias. Our findings may facilitate the development of novel therapeutic strategies for managing the risk of sudden unexpected death in epilepsy.
Copyright: © 2025 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Idiopathic (Genetic) Generalized Epilepsy.2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31536218 Free Books & Documents.
-
Seizure-related death exhibits a circadian rhythm independent of seizure timing or sleep in a mouse model of Dravet syndrome.J Physiol. 2025 Jun;603(12):3571-3587. doi: 10.1113/JP288697. Epub 2025 Jun 2. J Physiol. 2025. PMID: 40455956 Free PMC article.
-
Incidence and Types of Cardiac Arrhythmias in the Peri-Ictal Period in Patients Having a Generalized Convulsive Seizure.Neurology. 2024 Jul 9;103(1):e209501. doi: 10.1212/WNL.0000000000209501. Epub 2024 Jun 13. Neurology. 2024. PMID: 38870452 Free PMC article.
-
Circadian (diurnal/nocturnal) pattern of cardiac arrhythmias.Heart Rhythm. 2025 Aug;22(8):1994-2009. doi: 10.1016/j.hrthm.2024.10.013. Epub 2024 Oct 11. Heart Rhythm. 2025. PMID: 39395570 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Chahal CAA, Salloum MN, Alahdab F, Gottwald JA, Tester DJ, Anwer LA, et al. Systematic Review of the Genetics of Sudden Unexpected Death in Epilepsy: Potential Overlap With Sudden Cardiac Death and Arrhythmia-Related Genes. J Am Heart Assoc. 2020;9(1):e012264. doi: 10.1161/JAHA.119.012264 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

