Impact of ligand binding on VEGFR1, VEGFR2, and NRP1 localization in human endothelial cells
- PMID: 40668864
- PMCID: PMC12310042
- DOI: 10.1371/journal.pcbi.1013254
Impact of ligand binding on VEGFR1, VEGFR2, and NRP1 localization in human endothelial cells
Abstract
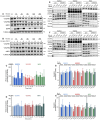
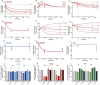
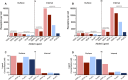
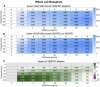
The vascular endothelial growth factor receptors (VEGFRs) bind to cognate ligands to facilitate signaling pathways critical for angiogenesis, the growth of new capillaries from existing vasculature. Intracellular trafficking regulates the availability of receptors on the cell surface to bind ligands, which regulate activation, and the movement of activated receptors between the surface and intracellular pools, where they can initiate different signaling pathways. Using experimental data and computational modeling, we recently demonstrated and quantified the differential trafficking of three VEGF receptors, VEGFR1, VEGFR2, and coreceptor Neuropilin-1 (NRP1). Here, we expand that approach to quantify how the binding of different VEGF ligands alters the trafficking of these VEGF receptors and demonstrate the consequences of receptor localization and ligand binding on the localization and dynamics of signal initiation complexes. We include simulations of four different splice isoforms of VEGF-A and PLGF, each of which binds to different combinations of the VEGF receptors, and we use new experimental data for two of these ligands to parameterize and validate our model. We show that VEGFR2 trafficking is altered in response to ligand binding, but that trafficking of VEGFR1 is not; we also show that the altered trafficking can be explained by a single mechanistic process, increased internalization of the VEGFR2 receptor when bound to ligand; other processes are unaffected. We further show that even though the canonical view of receptor tyrosine kinases is of activation on the cell surface, most of the ligand-receptor complexes for both VEGFR1 and VEGFR2 are intracellular. We also explore the competition between the receptors for ligand binding, the so-called 'decoy effect', and show that while in vitro on the cell surface minimal such effect would be observed, inside the cell the effect can be substantial and may influence signaling. We term this location dependence the 'reservoir effect' as the size of the local ligand reservoir (large outside the cell, small inside the cell) plays an integral role in the receptor-receptor competition. These results expand our understanding of receptor-ligand trafficking dynamics and are critical for the design of therapeutic agents to regulate ligand availability to VEGFR1 and hence VEGF receptor signaling in angiogenesis.
Copyright: © 2025 Sarabipour et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Anti-vascular endothelial growth factor biosimilars for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2024 Jun 3;6(6):CD015804. doi: 10.1002/14651858.CD015804.pub2. Cochrane Database Syst Rev. 2024. PMID: 38829176 Free PMC article.
-
Trafficking dynamics of VEGFR1, VEGFR2, and NRP1 in human endothelial cells.PLoS Comput Biol. 2024 Feb 7;20(2):e1011798. doi: 10.1371/journal.pcbi.1011798. eCollection 2024 Feb. PLoS Comput Biol. 2024. PMID: 38324585 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

