Multi-scale computational modeling towards efficacy in radiopharmaceutical therapies while minimizing side effects: Modeling of amino acid infusion
- PMID: 40668873
- PMCID: PMC12327665
- DOI: 10.1371/journal.pcbi.1013247
Multi-scale computational modeling towards efficacy in radiopharmaceutical therapies while minimizing side effects: Modeling of amino acid infusion
Abstract
Amino acid infusion (AAI) is a technique used in radiopharmaceutical therapy (RPT) to reduce toxicity in kidney and increase clearance rate of radiopharmaceuticals from body. In this study our aim is to evaluate its effect in personalized RPT considering kidney and salivary glands as dose limiting organs using a multiscale modeling framework. We developed a Physiologically-Based Pharmacokinetic (PBPK) model consisting of 19 compartments, personalized it for four prostate cancer patients using data derived from gamma camera imaging. This model was used to investigate the influence of AAI on the absorbed dose to tumors and organs at risk. We then computed the maximum safe injected activity based on the PBPK model. To address the effects of interstitial fluid pressure (IFP) and tumor heterogeneity, we coupled the PBPK model with convection-diffusion-reaction (CDR) equations. To compare the effectiveness of our modeling approaches, we calculated absorbed doses to the tumors with and without AAI, using both the standalone PBPK model and the coupled PBPK-CDR model. Our findings revealed a relative error (RE) of 9.6% ± 2.2% (mean ± SD) in total tumor absorbed dose calculation between PBPK and CDR equations, attributable to the consideration of IFP. Moreover, AAI proved beneficial for RPT when the kidney was designated as the organ-at-risk. It enabled an increase in radiopharmaceutical injection from 12.3 ± 6.32 MBq (mean ± SD) to 15.45 ± 6.95 MBq (RE: 28.5% ± 15.7%), resulting in a corresponding increase in tumor absorbed dose from 67.8 ± 47.45 Gy to 72.43 ± 51.03 Gy (RE: 8.6% ± 5.4%), while maintaining critical kidney absorbed dose limits. However, this was not observed when the salivary gland was considered the dose-limiting organ. Although, AAI allowed for increased therapeutic injection ranging from 4.22 ± 2.23 MBq to 5.25 ± 3.14 MBq (RE: 19.2% ± 9.9%), it results in a minimal increase in tumor absorbed dose of 0.22 ± 0.04 (RE: 1.4% ± 1.3%). Statistical analysis using the Wilcoxon Signed-Rank Test revealed significant effects of AAI on administered activity and tumor absorbed dose (p-value = 0.007 < 0.05). Finally, a local sensitivity analysis was performed on selected radiation and tumor transportation parameters individually to evaluate their impact on the tumor absorbed dose. In conclusion, selection of organ-at-risk in personalized RPT is critical, as it determines the injected activity amount and the efficacy of delivery-enhancing techniques.
Copyright: © 2025 Golzaryan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



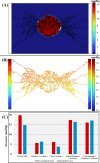
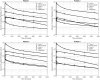
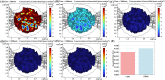



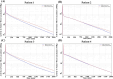
Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

