A forward-engineered, muscle-driven soft robotic swimmer
- PMID: 40668908
- PMCID: PMC12266098
- DOI: 10.1126/sciadv.adu8634
A forward-engineered, muscle-driven soft robotic swimmer
Abstract
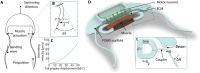
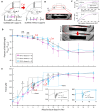
The field of biohybrid robotics focuses on using biological actuators to study the emergent properties of tissues and the locomotion of living organisms. On the basis of models of swimming at small size scales, we designed and fabricated a muscle-powered, flagellate swimmer. We investigate the design of a compliant mechanism based on nonlinear mechanics and its mechanical integration with a muscle ring and motor neurons. We find that within a range of anchor stiffnesses around 1 micronewton per micrometer, the homeostatic tension in muscle is insensitive to stiffness, offering greater design flexibility. The proximity of motor neurons results in a fourfold improvement in muscle contractility. Improved contractility and nonlinear design allow for a peak swimming speed about two orders of magnitude higher than previous biohybrid flagellate swimmers, reaching 0.58 body lengths per minute (86.8 micrometers per second), by a mechanism involving inertia that we verify through flow field imaging. This swimmer opens the door for a class of intermediate-Reynolds number swimmers.
Figures







References
-
- Webster-Wood V. A., “ It’s alive! From bioinspired to biohybrid robots,” in Robotics in Natural Settings. CLAWAR 2022. Lecture Notes in Networks and Systems, Cascalho J. M., Tokhi M. O., Silva M. F., Mendes A., Goher K., Funk M., Eds. (Springer, 2023), vol. 530, pp. 4.
-
- Mestre R., Patiño T., Sánchez S., Biohybrid robotics: From the nanoscale to the macroscale. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 13, e1703 (2021). - PubMed
-
- Filippi M., Mekkattu M., Katzschmann R. K., Sustainable biofabrication: From bioprinting to AI-driven predictive methods. Trends Biotechnol. 43, 290–303 (2025). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

