Validation of the Seizure-Related Impact Assessment Scale: A Novel Patient-Reported Outcome Measure for Epilepsy
- PMID: 40669025
- PMCID: PMC12264976
- DOI: 10.1212/WNL.0000000000213900
Validation of the Seizure-Related Impact Assessment Scale: A Novel Patient-Reported Outcome Measure for Epilepsy
Abstract
Background and objectives: There is a clear need in epilepsy clinical trials and practice for a measure that captures the trade-off between seizure and treatment-related adverse effects, which is reliable over time and across different treatment regimens. We aimed to create and validate the Seizure-Related Impact Assessment Scale (SERIAS) to fill this need.
Methods: This was a prospective longitudinal study of adults with epilepsy recruited from an Australian comprehensive epilepsy center. Participants completed SERIAS at baseline and 3 and 6 months later. SERIAS has 6 self-report items. Five items record the number of days per month that seizures or treatment-related adverse effects partially or fully affect work/home/school and family/social/nonwork activities. The final item is an epilepsy disability visual analog scale. SERIAS is scored by adding the days per month of disability, with scores ranging from 0 to 150 (higher scores indicate more disability). SERIAS was completed alongside 7 validated instruments measuring seizure-related and treatment-related adverse effects (Work and Social Adjustment Scale [WSAS], Liverpool Adverse Events Profile [LAEP]), mood disorders (Neurological Disorders Depression Inventory for Epilepsy [NDDI-E], Generalized Anxiety Disorder [GAD-7]), somatic symptoms (Somatic Symptom Scale [SSS-8]), and quality of life (Quality of Life in Epilepsy Inventory [QOLIE]-31, EuroQol 5 Dimensions [EQ-5D]). General linear mixed models were used to investigate the relationship between the SERIAS and other relevant clinical and psychometric data. Standardized model coefficients β are presented with 95% confidence intervals.
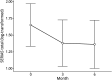
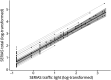
Results: A total of 90 patients (64.4% female, mean age 43.1 years) completed baseline SERIAS. Most patients reported at least 1 day of disability (62%, median SERIAS score = 3, interquartile range = 18.3). Greater disability was negatively correlated with QOLIE-31 total score (β = -0.17, 95% CI -0.27 to -0.07) and positively correlated with scores on 5-level EQ-5D (β = 0.15, 95% CI 0.04-0.25), NDDI-E (β = 0.22, 95% CI 0.13-0.31), GAD-7 (β = 0.21, 95% CI 0.09-0.32), SSS8 (β = 0.29, 95% CI 0.17-0.41), LAEP (β = 0.29, 95% CI 0.20-0.39), WSAS seizure-related adverse events (β = 0.23, 95% CI 0.14-0.33), and WSAS treatment-related adverse events (β = 0.36, 95% CI 0.26-0.46). Higher seizure frequency was associated with higher SERIAS score (β = 0.07, 95% CI 0.03-0.11). Psychometric reliability for the SERIAS was acceptable (all coefficients >0.70) as was test-retest reliability (n = 35 patients, intraclass correlation coefficient = 0.72, 95% CI 0.51-0.85).
Discussion: SERIAS shows good psychometric reliability and strong test-retest stability. These findings suggest that SERIAS is a valid scale to measure epilepsy-related disability.
Conflict of interest statement
E. Foster reports salary support from LivaNova USA for the SERIAS validation study; and she and her institution report grants from the Avant Research Foundation, Brain Foundation Australia, the Clive and Vera Ramaciotti Foundation (Ramaciotti Health Investment Grant), GPCE, LivaNova (Australia and USA), Lundbeck (Australia), Monash Partners STAR Clinician Fellowship, Monash University Early Career Postdoctoral Fellowship, Royal Australasian College of Physicians Fellows Research Establishment Fellowship, and the Sylvia and Charles Viertel Charitable Foundation Clinical Investigator Award, and UCB, outside the submitted work. A. Conquest reports salary support from LivaNova USA for the SERIAS validation study; and she and/or her institution declare research support from Brain Foundation (Australia), outside the submitted work. J.-P. Nicolo and/or his institution have received research support and/or consultancy fees from Eisai, outside the submitted work. T.J. O'Brien and/or his institution have received research support from Australian and US government research granting organisation (NHMRC Program Grant #APP1091593, MRFF, DOD, National Institute of Neurological Disorders and Stroke); and research and/or consultancy fees from Industry: Biogen, Eisai Pharma, Jazz Pharmaceuticals, LivaNova, ES Therapeutics, Kinosis Pharmaceuticals, Supernus, UCB Pharma, and Zynerba Pharmaceuticals, outside the submitted work. P. Kwan is supported by a NHMRC Investigator Grant (GNT2025849); and he and/or his institution have received research support or consultancy fees from Angelini, Eisai, Jazz Pharmaceuticals, LivaNova, SK Life Science, and UCB Pharma, outside the submitted work. C. Malpas reports salary support from LivaNova USA for the SERIAS validation study; has received conference travel support and/or speaker fees from Merck, Novartis, and Biogen, outside the submitted work; and has received research support from the National Health and Medical Research Council, Multiple Sclerosis Research Australia, the University of Melbourne, the Royal Melbourne Hospital Neuroscience Foundation, and Dementia Australia, outside the submitted work. J. French consults for LivaNova; is president of the Epilepsy Study Consortium that owns the copyright to SERIAS; receives salary support from the Epilepsy Foundation and from the Epilepsy Study Consortium for consulting work and/or attending scientific advisory boards for Acadia Pharmaceuticals, Access Industries, Acuta Capital Partners, AFASCI Inc., Agrithera Inc., Alterity Therapeutics Ltd., Angelini Pharma S.p.A, Autifony Therapeutics Ltd., Axonis Therapeutics, Baergic Bio, Beacon Biosignals Inc., Biogen, Biohaven Pharmaceuticals, Bloom Science Inc., Bright Minds Biosciences Inc., Camp4 Therapeutics Corporation, Capsida Biotherapeutics, Cerebral Therapeutics, Cerecin Inc., Cerevel, Ceribell, Cognizance Biomarkers, Cowen and Company, LLC, Crossject, EcoR1 Capital, Eisai, Encoded Therapeutics, Engrail, Epalex, EpiMinder, Epitel Inc., Equilibre BioPharmaceuticals, Genentech Inc., Grin Therapeutics, iQure Pharma Inc, IQVIA RDS Inc., Janssen Pharmaceutica, Jazz Pharmaceuticals, Korro Bio Inc., Leal Therapeutics Inc., Lipocine, LivaNova, Longboard Pharmaceuticals, Marinus, Modulight.bio, Neumirna Therapeutics, Neurelis, Neurocrine, NeuroPace Inc., NeuroPro Therapeutics, Neuroventis, Neurona Therapeutics, Ono Pharmaceutical Co., Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Praxis, PureTech LTY Inc., Rapport Therapeutics Inc., Receptor Holdings Inc., River0vest Venture Partners, Sage Therapeutics Inc., SK Life Sciences, Stoke, Supernus, Takeda, Taysha Gene Therapies, Third Rock Ventures LLC, UCB Inc., Ventus Therapeutics, Vida Ventures Management, and Xenon; and has received research support from the Epilepsy Study Consortium (funded by Eisai and UCB), the Epilepsy Study Consortium/Epilepsy Foundation, (funded by UCB), GW/FACES/One8Foundation, and the National Institute of Neurological Disorders and Stroke, outside the submitted work. All other authors report no relevant disclosures. Go to
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
