Prognostic impact and clinical management of pT4N0 colon cancer: data from a large, multicenter, international, real-world dataset
- PMID: 40669095
- PMCID: PMC12281219
- DOI: 10.1016/j.esmoop.2025.105496
Prognostic impact and clinical management of pT4N0 colon cancer: data from a large, multicenter, international, real-world dataset
Abstract
Background: T4 is one of the most important prognostic factors in localized colon cancer (CC), especially in stage II (pT4N0). However, the optimal adjuvant treatment in this subset of patients remains unclear. We present a large, multicenter, international, real-world analysis of pT4N0 CC patients.
Patients and methods: A real-world database regarding clinicopathological characteristics of patients with stage II pT4N0 CC surgically removed between 2010 and 2021 was queried. Primary endpoints were overall survival (OS) and relapse-free survival (RFS), and analyses were adjusted on age (with a cut-off of 75 years) to reduce selection bias.
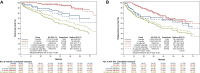
Results: Our study included 492 patients; outcomes data were available for 390 patients. Median age was 73 years. Microsatellite status was assessed in 294 (75%), including 74 (25%) mismatch repair deficient (dMMR)/microsatellite instability (MSI). Adjuvant chemotherapy was prescribed in 204 patients (52%), mostly oxaliplatin-based (70%). After a median follow-up of 46.8 months, 6 months of adjuvant chemotherapy was associated with a significant improvement in OS [hazard ratio (HR) age-adjusted 0.22, P < 0.001] when compared with no adjuvant. The benefit was seen also with 3 months of adjuvant chemotherapy, even if the benefit was lower (HR age-adjusted 0.60, P < 0.001). Similar results were observed in terms of RFS, with a statistically significant benefit both in the 6-month group (HR age-adjusted 0.47, P = 0.001) and in the 3-month group (HR age-adjusted 0.71, P = 0.001). Considering the regimen and the duration of treatment, 6 months of oxaliplatin-based chemotherapy was associated with a significant improvement in both OS and RFS (P < 0.001). In univariate analysis, MMR status was not associated with OS nor RFS.
Conclusions: T4 was confirmed to be a poor prognostic factor. Adjuvant chemotherapy provided a large benefit, with a significant reduction in risk of recurrence and death. The benefit was proportional to its duration, and oxaliplatin-based chemotherapy may be better than monotherapy.
Keywords: adjuvant chemotherapy; pT4N0; real-word dataset; stage II colon cancer.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure MAC reports travel support and hospitality from Pierre Fabre, Amgen, Merck, Servier, and Bayer, and has served on an advisory board for Merck. VF reports honoraria from Amgen, Merck, Servier, Merck Sharpe & Dohme (MSD), Bristol Myers Squibb (BMS), and Pierre Fabre. SS is an advisory board member for Agenus, AstraZeneca, Bayer, BMS, CheckmAb, Daiichi Sankyo, GSK, MSD, Merck, Novartis, Pierre Fabre, Pfizer, Seagen, and T-One Therapeutics. CS reports consultant fees from Amgen and Bayer. AS acted as speaker/consultant for Johnson & Johnson, Stryker, and Oasis. GT reports consulting or advisory roles for BMS, AstraZeneca, MSD, Merck, and Servier. JT has received honoraria as a speaker and/or in an advisory role from Amgen, Astellas, AstraZeneca, Boehringer, BMS, Brenus Pharma, Bicara Therapeutics, Oxford Biotherapeutics, Proskope, Merck KGaA, MSD, Novartis, ONO Pharmaceutical, Pierre Fabre, Natera, Sanofi, Servier, and Takeda. AP reports honoraria from GlaxoSmithKline, Takeda, Bayer, Daiichi Sankyo, MSD, Amgen, BeiOne, Pierre Fabre, Servier, BMS, Merck Serono for consultancy, advisory boards or invited speaker; research funding (to the Institution) from GlaxoSmithKline, Amgen; travel Grants from AstraZeneca. All outside the submitted work. LS reports consulting or advisory roles for Pierre Fabre, AstraZeneca, Bayer, Servier, Merck, Amgen, GSK, Incyte, LEO Pharma, MSD, and Takeda. All other authors have declared no conflicts of interest.
Figures

Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Assessment of the Addition of Oxaliplatin to Fluoropyrimidine-Based Adjuvant Chemotherapy in Patients With High-Risk Stage II Colon Cancer: An ACCENT Pooled Analysis.J Clin Oncol. 2024 Dec 10;42(35):4187-4195. doi: 10.1200/JCO.24.00394. Epub 2024 Sep 4. J Clin Oncol. 2024. PMID: 39231393
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. - PubMed
-
- Brierley J.D., Gospodarowicz M.K., Wittekind C. 8th ed. John Wiley; Oxford: 2016. TNM Classification of Malignant Tumours.
-
- Roth A.D., Delorenzi M., Tejpar S., et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635–1646. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

