Hypoxia, cuproptosis, and osteoarthritis: Unraveling the molecular crosstalk
- PMID: 40669206
- PMCID: PMC12283560
- DOI: 10.1016/j.redox.2025.103757
Hypoxia, cuproptosis, and osteoarthritis: Unraveling the molecular crosstalk
Abstract
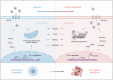
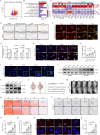
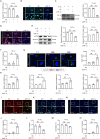
Aberrant changes in the hypoxic microenvironment are implicated in osteoarthritis (OA) development. Cuproptosis, a unique copper-dependent form of regulated cell death that depends on the activity of key enzymes in mitochondrial metabolism, is also linked to oxygen levels. However, the crosstalk among hypoxic environment, cuproptosis, and OA development remains unclear. This study confirmed that oxygen levels in the OA model gradually increased during OA progression, which suppressed the expression of anabolic genes for articular cartilage extracellular matrix, upregulated catabolic genes, and increased the cell death rate of primary chondrocytes. Mechanistically, oxygen elevation upregulated the expression of solute carrier family 31 member 1 (SCL31A1), a transmembrane pump facilitating copper uptake, whereas it downregulated the expression of ATPase copper transporting beta (ATP7B), a copper chaperone facilitating copper efflux, leading to aberrant copper accumulation in the cells. Ultimately, this accumulation efficiently induces oligomerization of dihydrolipoamide S-acetyltransferase (DLAT), triggering cell death, known as cuproptosis. Hypoxia-inducible factor-1α (HIF-1α), induced under hypoxic conditions, is negatively correlated with DLAT and the severity of OA, as confirmed in human and rat cartilage. Furthermore, siRNA-mediated HIF-1α silencing sensitized primary chondrocytes to cuproptosis, whereas HIF-1α stabilization had protective effects. In summary, increased oxygen levels in the cartilage induce cuproptosis, thereby accelerating OA progression, whereas HIF-1α stabilization mitigates this process. These findings may provide novel therapeutic targets for OA treatment in clinical practice.
Keywords: HIF-1α; Osteoarthritis; cuproptosis; hypoxic environment.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Maintaining high levels of HIF-1α protects osteoarthritis cartilage by activating autophagy.Tissue Cell. 2025 Oct;96:103027. doi: 10.1016/j.tice.2025.103027. Epub 2025 Jul 3. Tissue Cell. 2025. PMID: 40633452
-
Hypoxia-Inducible Factors (HIFs) in the articular cartilage: a systematic review.Eur Rev Med Pharmacol Sci. 2017 Jun;21(12):2800-2810. Eur Rev Med Pharmacol Sci. 2017. PMID: 28682438
-
Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α.Int J Mol Sci. 2025 Jul 2;26(13):6370. doi: 10.3390/ijms26136370. Int J Mol Sci. 2025. PMID: 40650148 Free PMC article.
-
Undercarboxylated OCN Inhibits Chondrocyte Hypertrophy and Osteoarthritis Development through GPRC6A/HIF-1α Cascade.Int J Biol Sci. 2025 Jun 23;21(10):4353-4373. doi: 10.7150/ijbs.105560. eCollection 2025. Int J Biol Sci. 2025. PMID: 40765832 Free PMC article.
-
Cuproptosis: a novel therapeutic mechanism in lung cancer.Cancer Cell Int. 2025 Jun 24;25(1):231. doi: 10.1186/s12935-025-03864-1. Cancer Cell Int. 2025. PMID: 40555995 Free PMC article. Review.
References
-
- Zhu J., Yang S., Qi Y., Gong Z., Zhang H., Liang K., Shen P., Huang Y.Y., Zhang Z., Ye W., Yue L., Fan S., Shen S., Mikos A.G., Wang X., Fang X. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci. Adv. 2022;8(13) - PMC - PubMed
-
- van de Stadt L.A., Haugen I.K., Felson D., Kloppenburg M. Prolonged morning stiffness is common in hand OA and does not preclude a diagnosis of hand osteoarthritis. Osteoarthr. Cartil. 2023;31(4):529–533. - PubMed
-
- Wang T., He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

