Automatic segmentation of liver structures in multi-phase MRI using variants of nnU-Net and Swin UNETR
- PMID: 40670420
- PMCID: PMC12267559
- DOI: 10.1038/s41598-025-07084-5
Automatic segmentation of liver structures in multi-phase MRI using variants of nnU-Net and Swin UNETR
Abstract
Accurate segmentation of the liver parenchyma, portal veins, hepatic veins, and lesions from MRI is important for hepatic disease monitoring and treatment. Multi-phase contrast enhanced imaging is superior in distinguishing hepatic structures compared to single-phase approaches, but automated approaches for detailed segmentation of hepatic structures are lacking. This study evaluates deep learning architectures for segmenting liver structures from multi-phase Gd-EOB-DTPA-enhanced T1-weighted VIBE MRI scans. We utilized 458 T1-weighted VIBE scans of pathological livers, with 78 manually labeled for liver parenchyma, hepatic and portal veins, aorta, lesions, and ascites. An additional dataset of 47 labeled subjects was used for cross-scanner evaluation. Three models were evaluated using nested cross-validation: the conventional nnU-Net, the ResEnc nnU-Net, and the Swin UNETR. The late arterial phase was identified as the optimal fixed phase for co-registration. Both nnU-Net variants outperformed Swin UNETR across most tasks. The conventional nnU-Net achieved the highest segmentation performance for liver parenchyma (DSC: 0.97; 95% CI 0.97, 0.98), portal vein (DSC: 0.83; 95% CI 0.80, 0.87), and hepatic vein (DSC: 0.78; 95% CI 0.77, 0.80). Lesion and ascites segmentation proved challenging for all models, with the conventional nnU-Net performing best. This study demonstrates the effectiveness of deep learning, particularly nnU-Net variants, for detailed liver structure segmentation from multi-phase MRI. The developed models and preprocessing pipeline offer potential for improved liver disease assessment and surgical planning in clinical practice.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

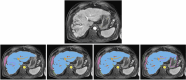
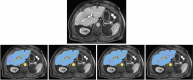
Similar articles
-
Large-scale convolutional neural network for clinical target and multi-organ segmentation in gynecologic brachytherapy via multi-stage learning.Med Phys. 2025 Aug;52(8):e18067. doi: 10.1002/mp.18067. Med Phys. 2025. PMID: 40817425
-
Comprehensive Segmentation of Gray Matter Structures on T1-Weighted Brain MRI: A Comparative Study of Convolutional Neural Network, Convolutional Neural Network Hybrid-Transformer or -Mamba Architectures.AJNR Am J Neuroradiol. 2025 Apr 2;46(4):742-749. doi: 10.3174/ajnr.A8544. AJNR Am J Neuroradiol. 2025. PMID: 39433334
-
A full-scale attention-augmented CNN-transformer model for segmentation of oropharyngeal mucosa organs-at-risk in radiotherapy.Phys Eng Sci Med. 2025 Sep 11. doi: 10.1007/s13246-025-01614-1. Online ahead of print. Phys Eng Sci Med. 2025. PMID: 40932560
-
Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2013 Apr;17(16):1-243. doi: 10.3310/hta17160. Health Technol Assess. 2013. PMID: 23611316 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
References
-
- Wilson, G. C. et al. Texture analysis on preoperative contrast-enhanced magnetic resonance imaging identifies microvascular invasion in hepatocellular carcinoma. HPB22, 1622–1630. 10.1016/j.hpb.2020.03.001 (2020). - PubMed
-
- Wei, J. et al. Radiomics: A radiological evidence-based artificial intelligence technique to facilitate personalized precision medicine in hepatocellular carcinoma. Dig. Liver Dis.55, 833–847. 10.1016/j.dld.2022.12.015 (2023). - PubMed
-
- Martí-Aguado, D. et al. Automated whole-liver MRI segmentation to assess steatosis and iron quantification in chronic liver disease. Radiology302, 345–354. 10.1148/radiol.2021211027 (2022) (PMID: 34783592). - PubMed
-
- Ronneberger, O., Fischer, P. & Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015 (eds Navab, N. et al.) 234–241 (Springer, 2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

