Evaluating the impact of denoising diffusion MRI data on tractometry metrics of optic tract abnormalities in glaucoma
- PMID: 40670518
- PMCID: PMC12267541
- DOI: 10.1038/s41598-025-10947-6
Evaluating the impact of denoising diffusion MRI data on tractometry metrics of optic tract abnormalities in glaucoma
Abstract
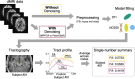
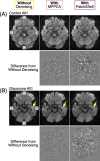
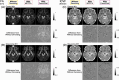
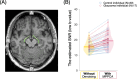
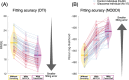
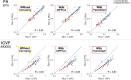
Diffusion MRI (dMRI)-based tractometry is a non-invasive neuroimaging method for evaluating white matter tracts in living humans, capable of detecting abnormalities caused by disorders. However, measurement noise in dMRI data often compromises the signal quality. Several denoising methods for dMRI have been proposed, but the extent to which denoising affects tractometry metrics of white matter tissue properties associated with disorders remains unclear. We evaluated how denoising affects tractometry along the optic tract (OT) in patients with glaucoma. Because glaucoma damages retinal ganglion cells, the OT in patients with glaucoma is likely to exhibit tissue abnormalities. Therefore, we examined dMRI data from patients with glaucoma to evaluate how two widely used denoising methods (MPPCA and Patch2Self) affect tractometry metrics regarding the expected tissue changes in the OT. We found that denoising affected the appearance of diffusion-weighted images, increased the estimated signal-to-noise ratio, and reduced residuals in voxelwise model fitting. However, denoising had a limited impact on the differences in tractometry metrics of the OT between patients with glaucoma and controls. Moreover, we found no evidence that denoising improved the reproducibility of tractometry. These findings suggest that the current denoising methods have a limited impact when used together with a tractometry framework.
Keywords: Denoising; Diffusion MRI; Glaucoma; Optic tract; Tractometry; White matter.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Denoising Improves Cross-Scanner and Cross-Protocol Test-Retest Reproducibility of Diffusion Tensor and Kurtosis Imaging.Hum Brain Mapp. 2025 Mar;46(4):e70142. doi: 10.1002/hbm.70142. Hum Brain Mapp. 2025. PMID: 40051327 Free PMC article.
-
Microstructural mapping of neural pathways in Alzheimer's disease using macrostructure-informed normative tractometry.Alzheimers Dement. 2025 Jan;21(1):e14371. doi: 10.1002/alz.14371. Epub 2024 Dec 30. Alzheimers Dement. 2025. PMID: 39737627 Free PMC article.
-
Histological validation of per-bundle water diffusion metrics within a region of fiber crossing following axonal degeneration.Neuroimage. 2019 Nov 1;201:116013. doi: 10.1016/j.neuroimage.2019.116013. Epub 2019 Jul 19. Neuroimage. 2019. PMID: 31326575
-
Optic nerve head and fibre layer imaging for diagnosing glaucoma.Cochrane Database Syst Rev. 2015 Nov 30;2015(11):CD008803. doi: 10.1002/14651858.CD008803.pub2. Cochrane Database Syst Rev. 2015. PMID: 26618332 Free PMC article.
-
Surgical interventions for bilateral congenital cataract in children aged two years and under.Cochrane Database Syst Rev. 2022 Sep 15;9(9):CD003171. doi: 10.1002/14651858.CD003171.pub3. Cochrane Database Syst Rev. 2022. PMID: 36107778 Free PMC article.
References
-
- Ramrattan, R. S. et al. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: the Rotterdam Study. Arch. Ophthalmol.119, 1788–1794 (2001). - PubMed
-
- Iwase, A., Suzuki, Y., Araie, M. & Tajimi Study Group. Characteristics of undiagnosed primary open-angle glaucoma: the Tajimi Study. Ophthalmic Epidemiol.21, 39–44 (2014). - PubMed
-
- Gupta, N. & Yücel, Y. H. What changes can we expect in the brain of glaucoma patients? Surv. Ophthalmol.52 (Suppl 2), S122–S126 (2007). - PubMed
-
- Jones, D. K., Travis, A. R., Eden, G., Pierpaoli, C. & Basser, P. J. PASTA: Pointwise assessment of streamline tractography attributes. Magn. Reson. Med.53, 1462–1467 (2005). - PubMed
MeSH terms
Grants and funding
- JP20K18396/the Japan Society for the Promotion of Science (JSPS) KAKENHI
- JP22K09841/the Japan Society for the Promotion of Science (JSPS) KAKENHI
- 23NIPS141/the Cooperative Study Program of the National Institute for Physiological Sciences
- JPMXP1323015488/the MEXT Promotion of Development of a Joint Usage/Research System Project: Coalition of Universities of Research Excellence Program (CURE)
LinkOut - more resources
Full Text Sources
Medical

