The impact of vaccine booster doses on specific B- and T-lymphocyte dynamics in Thai healthcare personnel following COVID-19 vaccination
- PMID: 40670524
- PMCID: PMC12267843
- DOI: 10.1038/s41598-025-10400-8
The impact of vaccine booster doses on specific B- and T-lymphocyte dynamics in Thai healthcare personnel following COVID-19 vaccination
Abstract
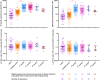
A primary series of Sinovac COVID-19 vaccine (CoronaVac) and ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine successfully increased anti-spike antibodies, neutralizing antibodies, and T-lymphocytes, as was also observed following booster doses of Oxford-AstraZeneca or BNT162b2 (Pfizer-BioNTech) vaccine. However, information regarding the dynamics of specific B- and T-lymphocytes induced by additional vaccinations remains limited. We examined the dynamics of specific B- and T-lymphocyte subsets induced by primary series vaccinations and booster doses over a two-year period of COVID-19 vaccination among healthcare personnel (HCP) enrolled in a prospective cohort study in Thailand. HCP, recruited between January and March 2021, had blood specimens collected at enrollment and at three-month intervals for cellular immune response testing. COVID-19 vaccinated participants (verified against documentation) were grouped by vaccination schedules: (A) CoronaVac with Oxford-AstraZeneca vaccine as the first booster dose (n = 46), (B) CoronaVac with Pfizer-BioNTech vaccine as the first booster dose (n = 53), and (C) Oxford-AstraZeneca vaccine (n = 29). All three groups had up to four subsequent booster doses of either the same or different platforms. Following the B-lymphocyte enzyme-linked immunospot and the T-lymphocyte intracellular cytokine staining assays, SARS-CoV-2 spike 1 (S1)- and receptor-binding domain (RBD)-specific antibody-secreting B-lymphocytes, and Interferon Gamma (IFN-Ƴ)- and/or Tumor Necrosis Factor Alpha (TNF-α)-producing T-lymphocytes for all blood collection time points were counted. Among participants without evidence of infection (i.e., those who tested negative for SARS-CoV-2 antibodies prior to vaccination and those who tested negative by SARS-CoV-2 real-time reverse transcription polymerase chain reaction during the study), levels of cellular immune response during weeks 1-12 since the last vaccine dose were compared between vaccine doses using the Kruskal-Wallis test. In all three groups, compared to the primary series, the first booster dose induced significant SARS-CoV-2 antigen-specific antibody-secreting B-lymphocyte counts (range 4.2-9.0-fold increase) but non-significant S1-specific cytokine-producing T-lymphocyte counts (range 0.5-1.3-fold). There were no notable differences in both antigen-specific antibody-secreting B-lymphocyte and specific cytokine-producing T-lymphocyte counts following the second, third, and fourth booster doses in all three groups compared to the first booster dose. Initial COVID-19 booster doses were essential for overall increases in the peak counts of antigen-specific B-lymphocytes, prior to minimal contraction phases occurred following additional boosters, while antigen-specific T-lymphocyte counts maintained a consistently high levels of immune response. The second, third, and fourth booster doses restored the levels of both B- and T-lymphocytes after the immune responses waned in a time-dependent manner.
Keywords: Antibody-secreting B-lymphocyte; Booster dose; COVID-19 vaccination; Cytokine-producing T-lymphocyte; SARS-CoV-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors have no conflicts of interest to declare. Ethical approval and consent to participate: This study was approved by the Institutional Review Boards (IRBs) of Department of Disease Control of the Thai Ministry of Public Health and four participating hospitals (Bamrasnaradura Infectious Diseases Institute; Phaholpolpayuhasena hospital; Phramongkutklao hospital [PMK], and Rayong hospital). The IRBs of the U.S. Centers for Disease Control and Prevention and Mahidol University relied on the determinations made by the PMK’s and MOPH’s IRBs, respectively. All participants provided written informed consent to participate.
Figures


Similar articles
-
A 20-month longitudinal study to evaluate humoral and cellular immunity after COVID-19 vaccines.Mem Inst Oswaldo Cruz. 2025 Jul 18;120:e240193. doi: 10.1590/0074-02760240193. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40699035 Free PMC article.
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
-
Mapping of human monoclonal antibody responses to XBB.1.5 COVID-19 monovalent vaccines: a B cell analysis.Lancet Microbe. 2025 Aug;6(8):101103. doi: 10.1016/j.lanmic.2025.101103. Epub 2025 May 30. Lancet Microbe. 2025. PMID: 40456237
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Functional antibody responses to SARS-CoV-2 variants before and after booster vaccination among adults in Ghana.Exp Biol Med (Maywood). 2025 Jul 21;250:10440. doi: 10.3389/ebm.2025.10440. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 40761773 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

