"Injectable ARVs will give me peace" women's acceptability of injectable antiretroviral therapy in prevention of HIV vertical transmission in Uganda
- PMID: 40670534
- PMCID: PMC12267473
- DOI: 10.1038/s41598-025-10686-8
"Injectable ARVs will give me peace" women's acceptability of injectable antiretroviral therapy in prevention of HIV vertical transmission in Uganda
Abstract
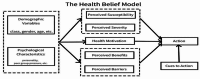
A third of women who initiate or continue antiretroviral (ARV) treatment during pregnancy are lost- to- follow-up (LTFU) within six months in sub-Saharan Africa. Clinical trials of long acting injectable Antiretroviral Therapy (LA-ART) are ongoing, including for pregnant women. The study explored women's acceptability and attitudes towards- the use of potentially discreet LA-ART in Uganda for Prevention of Vertical Transmission Program (PVT). Understanding women's perspectives of LA-ART before its introduction is critical in answering questions about acceptability and identifying interventions for future implementation and uptake. In May 2022, we purposively selected participants for eight focus group discussions (FGDs) in 5 health facilities in Kampala: 5 with retained mothers and 3 with disengaged mothers from the PVT. A woman was categorized as retained if she had at least one clinic encounter within her last visit. A total of two in-depth interviews were conducted with disengaged mothers. Luganda, the most widely spoken language in Kampala was used to conduct the FGDs and interviews. Recordings were transcribed and translated into English. Themes were mapped onto some components of the health belief model. NVivo version 14 software was used for data management. A thematic approach was used for analysis and a team-based approach was utilized. The data realized three broad themes (1) Perceived benefits from the use of the LA-ART like emotional relief from daily drugs, effective viral load suppression, reduction on stigma and discrimination, reduction of LTFU, (2) potential threats to rollout due to fear of side-effects from monthly injections, drug stock outs, the cost of LA-ART, myths about LA-ART that it cures HIV and (3) Approaches to increase access to LA-ART like prior investigations, providing bridging treatments and use peer-peer approaches. Most women in this study appeared to have positive attitudes towards the rollout of the LA-ART. LA-ART is a potential solution to LTFU which could improve retention in care for women living with HIV. To optimize the acceptability, health programs should ensure strengthened counseling and create awareness of LA-ART and use of peer- peer approaches with expert women using educational initiatives to raise awareness among other women.
Keywords: Acceptability; HIV treatment; Long-acting injectable antiretroviral; Prevention of vertical transmission; Qualitative study Uganda; Women living with HIV.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki. Ethics approval was obtained from the Infectious Diseases Institute (IDIREC) on 3 November 2021 (IDIREC 032/2021). Additional approvals were obtained from the Uganda National Council for Science and Technology (SS1095ES) on 8/12 2021. UCSF approval (Ref 327715) in 05/04/2022. All participants signed an informed consent sheet either in English or Luganda and confidentiality was maintained by anonymizing participants’ identifiers at all stages of data collection and reporting. All data were stored on encrypted computers. Filed notes and signed participant-informed consent forms were kept in a locked drawer at the study site. Participants’ names were not recorded anywhere during data collection. Each participant was given a unique identifying number to ensure confidentiality. The research teams did not include any identifying information that could have harmful consequences for the participants. Consent for publication: All authors reviewed approved the final manuscript and consented to submission for publication.
References
-
- Aizire, J., Fowler, G. & Coovadia, M. M. Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. Curr. HIV Res.11 (2), 144–159 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical