Evidence for trans-synaptic propagation of oligomeric tau in human progressive supranuclear palsy
- PMID: 40670683
- PMCID: PMC12321572
- DOI: 10.1038/s41593-025-01992-5
Evidence for trans-synaptic propagation of oligomeric tau in human progressive supranuclear palsy
Abstract
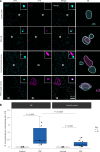
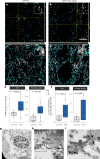
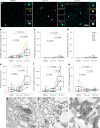
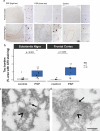
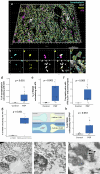
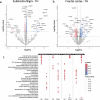
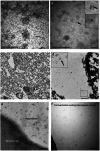
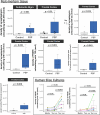
In the neurodegenerative disease progressive supranuclear palsy (PSP), tau pathology progresses through the brain in a stereotypical spatiotemporal pattern, and where tau pathology appears, synapses are lost. We tested the hypothesis that pathological tau contributes to synapse loss and may spread through the brain by moving from presynapses to postsynapses. Using postmortem PSP brain samples and a living human brain slice culture model, we observe pathological tau in synaptic pairs and evidence that oligomeric tau can enter live human postsynapses. Proteomics revealed increased clusterin in synapses in PSP, and super-resolution imaging showed clusterin colocalized with tau in synapses in close enough proximity to be binding partners, which may mediate tau spread. Accumulation of tau in synapses correlated with synapse loss, and synaptic engulfment by astrocytes was observed, suggesting that astrocytes contribute to synapse loss. Together, these data indicate that targeting synaptic tau is a promising approach to treat PSP.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.L.S.-J. is a scientific advisory board member of Scottish Brain Sciences, Cognition Therapeutics and Race Against Dementia, and has received consulting fees or honoraria from Jay Therapeutics, Sanofi, AbbVie, Merck and Eisai. M.T. is an employee of Scottish Brain Sciences. The other authors declare no competing interests.
Figures






References
-
- Steele, J. C., Richardson, J. C. & Olszewski, J. Progressive supranuclear palsy: a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch. Neurol.10, 333–359 (1964). - PubMed
-
- Bigio, E. H. et al. Cortical synapse loss in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol.60, 403–410 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

