CLDN18.2-targeting antibody-drug conjugate IBI343 in advanced gastric or gastroesophageal junction adenocarcinoma: a phase 1 trial
- PMID: 40670773
- PMCID: PMC12443601
- DOI: 10.1038/s41591-025-03783-8
CLDN18.2-targeting antibody-drug conjugate IBI343 in advanced gastric or gastroesophageal junction adenocarcinoma: a phase 1 trial
Abstract
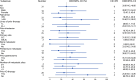
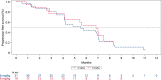
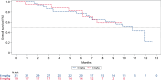
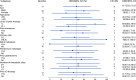
Aberrant expression of claudin18.2 (CLDN18.2) has frequently been observed in gastric and gastroesophageal junction (G/GEJ) adenocarcinoma, making it a promising therapeutic target for this aggressive cancer. While a monoclonal antibody targeting CLDN18.2 has been approved for G/GEJ adenocarcinoma, antibody-drug conjugates (ADCs) have also emerged as therapeutic modalities. IBI343 is an ADC consisting of a fully humanized anti-CLDN18.2 monoclonal antibody conjugated to exatecan via site-specific glycol conjugation and a cleavable linker with a drug-to-antibody ratio of 4. Here we present the results from a phase 1 dose escalation and dose expansion study of the IBI343 ADC. A total of 127 patients were enrolled and dosed (19 in the escalation phase and 108 in the expansion phase). Dose-limiting toxicities occurred in two of six participants at a dose of 10 mg kg-1, including one with myelosuppression (grade 4) and one with both neutropenia (grade 4) and febrile neutropenia (grade 3). Minimal gastrointestinal adverse events (grade ≥3) were observed and no interstitial lung disease was reported. The recommended phase 2 dose of IBI343 was determined to be 6 mg kg-1 every 3 weeks with a confirmed objective response rate of 29% and median progression-free survival of 5.5 months in CLDN18.2-high (2+/3+ ≥ 75%) G/GEJ adenocarcinoma. IBI343 was well tolerated, with a manageable safety profile and promising efficacy in G/GEJ adenocarcinoma. Further research is required to understand optimal sequencing, and biomarker-informed combination therapy, in G/GEJ tumors given the development of multiple therapies targeting CLDN18.2 in addition to human epidermal growth factor receptor 2 and programmed cell death 1 ligand 1.ClinicalTrials.gov registration: NCT05458219 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.L. declares honoraria from Merck Sharp & Dohme and Specialised Therapeutics, consulting or advisory roles for Starpharma and Greywolf Therapeutics, research funding from Starpharma, ViroCure, Corvus Pharmaceuticals, Relay Therapeutics, ALX Oncology, IDEAYA Biosciences, Innovent Biologics, Greywolf Therapeutics, Merck Sharp & Dohme, Regeneron, Bristol Myers Squibb, AbbVie and AVEO, and travel, accommodation and expenses from Starpharma, ImmVirX, Merck Sharp & Dohme and Innovent Biologics. Y.L., X.Z., Y.G. and H.Z. are employees of Innovent Biologics. The other authors declare no competing interests.
Figures



References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). - PubMed
-
- Huang, J. et al. Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol.20, 271–287 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

