A male-essential miRNA is key for avian sex chromosome dosage compensation
- PMID: 40670784
- PMCID: PMC12408383
- DOI: 10.1038/s41586-025-09256-9
A male-essential miRNA is key for avian sex chromosome dosage compensation
Abstract
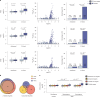
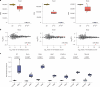
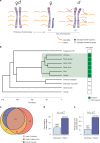
Birds have a sex chromosome system in which females are heterogametic (ZW) and males are homogametic (ZZ)1. The differentiation of avian sex chromosomes from ancestral autosomes entails the loss of most genes from the W chromosome during evolution1,2. However, the extent to which mechanisms evolved that counterbalance this substantial reduction in female gene dosage remains unclear. Here we report functional in vivo and evolutionary analyses of a Z-linked microRNA (miR-2954) with strong male-biased expression, previously proposed to mediate avian sex chromosome dosage compensation3. We knocked out miR-2954 in chicken, which resulted in early embryonic lethality in homozygous knockout males, probably driven by specific upregulation of dosage-sensitive Z-linked target genes. Evolutionary gene expression analyses further revealed that these dosage-sensitive target genes underwent both transcriptional and translational upregulation on the single Z in female birds. Altogether, this work unveils a scenario in which evolutionary pressures following W gene loss drove transcriptional and translational upregulation of dosage-sensitive Z-linked genes in females but also their transcriptional upregulation in males. The resulting excess of transcripts in males, resulting from the combined activity of two upregulated dosage-sensitive Z gene copies, was in turn offset by the emergence of a highly targeted miR-2954-mediated transcript degradation mechanism during avian evolution. This study uncovered a unique sex chromosome dosage compensation system in birds, in which a microRNA has become essential for male survival.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

