Feasibility and acceptability of conducting a birth cohort study during the COVID-19 pandemic: a mixed-methods study
- PMID: 40670909
- PMCID: PMC12265286
- DOI: 10.1186/s12884-025-07850-3
Feasibility and acceptability of conducting a birth cohort study during the COVID-19 pandemic: a mixed-methods study
Abstract
Background: “BABY1000” was a pilot prospective birth cohort study based in Sydney, Australia, which aimed to identify factors before and during pregnancy that impact child and long-term health and assess study feasibility and acceptability. The COVID-19 pandemic and associated public health orders affected study protocols. This sub-study aimed to explore feasibility and acceptability of BABY1000 within this context.
Methods: Recruitment of women commenced before conception or during early pregnancy attending clinics at a major hospital in Sydney. Data collection extended from recruitment, across pregnancy at 12-, 20-, 28- and 36-weeks’ gestation, and postnatally (in both mothers and children) until the child’s second birthday. Feasibility was assessed using routinely collected data on recruitment, retention, and completion. BABY1000 participants were invited to complete an online acceptability survey, including the State-Trait Anxiety Inventory, and to participate in focus group discussions. Paired t-tests were used to compare sub-study respondents to the wider cohort of participants to assess for demographic drivers of feasibility. Chi-squared tests were used to examine associations between high anxiety and demographic characteristics, study acceptability, and the perceived impact of COVID-19 on study participation.
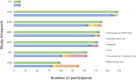
Results: From 225 women recruited, 180 (80%) remained enrolled at the end of pregnancy, with 100 (44%) remaining in the study until 24-months. Participant retention and data completeness were most challenging after birth. Eighty-seven mothers completed the acceptability survey and 22 parents participated in focus groups. Most participants found the study protocol to be acceptable and comfortable. A “high” anxiety score was not associated with study acceptability or willingness to participate, although metrics of both feasibility and acceptability were negatively impacted by the COVID-19 pandemic.
Conclusions: Whilst public health orders associated with the COVID-19 pandemic impacted feasibility and acceptability of the BABY1000 cohort, this sub-study found it is both feasible and acceptable to collect comprehensive biological, questionnaire, and health data from early pregnancy to two years. Study designs which tailor resources to where participant attrition can be predicted and have capacity to adapt to changing circumstances will be best placed to contribute to understanding of the Developmental Origins of Health and Disease.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12884-025-07850-3.
Keywords: Acceptability; COVID-19; Cohort studies; DOHaD; Feasibility; Pandemic; Pregnancy.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The BABY1000 pilot obtained ethical approval from Sydney Local Health District (RPAH zone) Review Committee (protocol no. X170019) and all procedures were followed in accordance with the Declaration of Helsinki. This sub-study received additional ethics approval to include questions relating to the impact of the COVID-19 pandemic and FGDs. All parents who agreed to participate were provided an information sheet and were required to indicate that they had read it before providing written consent. Whilst there were no anticipated harms of participating, the information also signposted relevant support information and services if participants felt distressed by sharing their experiences. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Socioemotional Development of Infants and Toddlers During the COVID-19 Pandemic.JAMA Pediatr. 2024 Feb 1;178(2):151-159. doi: 10.1001/jamapediatrics.2023.5684. JAMA Pediatr. 2024. PMID: 38147322 Free PMC article.
-
The impact of mitigation measures on perinatal outcomes during the first nine months of the COVID-19 pandemic: A systematic review with meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2022 Jul;274:117-127. doi: 10.1016/j.ejogrb.2022.05.007. Epub 2022 May 14. Eur J Obstet Gynecol Reprod Biol. 2022. PMID: 35640440 Free PMC article.
-
Association Between COVID-19 During Pregnancy and Preterm Birth by Trimester of Infection: A Retrospective Cohort Study Using Longitudinal Social Media Data.medRxiv [Preprint]. 2023 Nov 21:2023.11.17.23298696. doi: 10.1101/2023.11.17.23298696. medRxiv. 2023. Update in: J Med Internet Res. 2025 Jul 9;27:e66097. doi: 10.2196/66097. PMID: 38045356 Free PMC article. Updated. Preprint.
-
Feasibility and acceptability of a telephone-based ACT intervention for caregivers (TACTICs) of adults with Alzheimer's disease and related dementias (ADRD): a randomized pilot during the COVID-19 pandemic.Aging Ment Health. 2025 Apr;29(4):639-650. doi: 10.1080/13607863.2025.2453823. Epub 2025 Jan 21. Aging Ment Health. 2025. PMID: 39838852 Clinical Trial.
-
Impacts of COVID-19 pandemic on preterm birth: a systematic review and meta-analysis.Public Health. 2022 Dec;213:127-134. doi: 10.1016/j.puhe.2022.10.015. Epub 2022 Oct 19. Public Health. 2022. PMID: 36410118 Free PMC article.
References
-
- Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951–70. - PubMed
-
- Hagemann E, Silva DT, Davis JA, et al. Developmental origins of health and disease (DOHaD): the importance of life-course and transgenerational approaches. Paediatr Respir Rev. 2021;40:3–9. - PubMed
LinkOut - more resources
Full Text Sources

