Metformin facilitates osteogenic differentiation of bone marrow stromal cells through AMPK-dependent autophagy: an investigation into the healing of osteoporotic fractures in murine models
- PMID: 40671012
- PMCID: PMC12265109
- DOI: 10.1186/s13018-025-06067-6
Metformin facilitates osteogenic differentiation of bone marrow stromal cells through AMPK-dependent autophagy: an investigation into the healing of osteoporotic fractures in murine models
Abstract
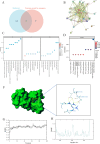
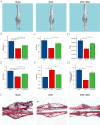
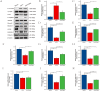
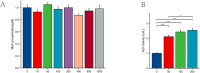
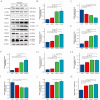
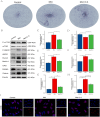
Osteoporosis is a widespread metabolic bone disorder characterized by a reduction in bone density and structural deterioration, leading to an increased susceptibility to fractures. This study investigates the role of metformin (Met) in promoting the osteogenic differentiation of bone marrow stromal cells (BMSCs) through the activation of AMP-activated protein kinase (AMPK) and explores its potential application in the treatment of osteoporotic fractures. We conducted a series of in vivo and in vitro experiments to elucidate the mechanisms underlying the effects of metformin. In a murine model of osteoporosis, metformin treatment significantly enhanced tibia fracture healing, as evidenced by increased bone mineral density (BMD), bone volume fraction (BV/TV), trabecular number (Tb.N), and decreased trabecular separation (Tb.Sp), as observed through micro-computed tomography (Micro-CT) and histological analyses. Immunoblot and real-time PCR demonstrated that metformin upregulated collagen type I (Col-I), a key osteogenic marker, and osteoprotegerin (OPG), an inhibitor of osteoclast differentiation that contributes to bone homeostasis via activated the AMPK signaling pathway. In vitro, metformin enhanced the osteogenic differentiation of BMSCs, as indicated by elevated alkaline phosphatase (ALP) activity. Western blot and PCR analyses further revealed that metformin increased the expression of AMPK, phosphorylated AMPK (p-AMPK), mammalian target of rapamycin (mTOR), phosphorylated mTOR (p-mTOR), Beclin-1, and LC3-II/LC3-I, suggesting enhanced autophagy. The application of the AMPK inhibitor Compound C attenuated these effects, confirming the role of AMPK-mediated autophagy in metformin-induced osteogenesis. These findings suggest that metformin, through the activation of AMPK and subsequent enhancement of autophagy, promotes the osteogenic differentiation of BMSCs and accelerates the healing of osteoporotic fractures. Future research should focus on optimizing metformin dosage and administration routes and evaluating the long-term safety and efficacy of this therapeutic approach for patients with osteoporotic fractures.
Supplementary Information: The online version contains supplementary material available at 10.1186/s13018-025-06067-6.
Keywords: AMPK; Autophagy; Metformin; Osteoblast differentiation; Osteoporotic fracture.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Eastell R, O’Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Reviews Disease Primers. 2016;2:16069. - PubMed
-
- Li Q, Zhang W, Deng J, et al. Ameliorative effects of extracellular vesicles derived from mesenchymal stem cells on apoptosis and differentiation of osteoblasts treated with CoCl(2). Cell Reprogram. 2023;25:99–108. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

