UCP2 inhibition exaggerates diabetic cardiomyopathy by facilitating the activation of NLRP3 and pyroptosis
- PMID: 40671041
- PMCID: PMC12269110
- DOI: 10.1186/s13098-025-01855-w
UCP2 inhibition exaggerates diabetic cardiomyopathy by facilitating the activation of NLRP3 and pyroptosis
Abstract
Background: Uncoupling protein 2 (UCP2) is implicated in cardiomyocyte apoptosis and diabetes, yet its role in diabetic cardiomyopathy (DCM) remains unclear. This study aimed to elucidate the mechanisms by which UCP2 influences DCM pathogenesis.
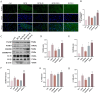
Methods: We examined UCP2 expression in DCM mice and assessed cardiac function via echocardiography. Myocardial fibrosis and hypertrophy were evaluated using Masson trichrome staining and wheat germ agglutinin (WGA) staining. The mitochondrial-targeted reactive oxygen species (ROS) scavenger mito-TEMPO was used to investigate the role of ROS. Mechanistic studies were conducted in H9C2 cells, focusing on ROS production, mitochondrial membrane potential, and the thioredoxin-interacting protein (TXNIP)/NLR family pyrin domain containing 3 (NLRP3)/gasdermin D (GSDMD) pathway.
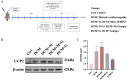
Results: UCP2 was upregulated in DCM mice hearts, and its inhibition worsened cardiac function, increased myocardial fibrosis, and aggravated cardiomyocyte hypertrophy. UCP2 knockdown in H9C2 cells elevated ROS levels, reduced mitochondrial membrane potential, and activated the TXNIP/NLRP3/GSDMD pathway, leading to pyroptosis. Mito-TEMPO partially reversed these effects by reducing ROS and suppressing NLRP3 and pyroptosis-related proteins.
Conclusions: UCP2 inhibition exacerbates DCM by inducing NLRP3- and GSDMD-mediated pyroptosis via the ROS/TXNIP axis. These findings offer new insights into DCM pathogenesis and potential therapeutic targets.
Keywords: Diabetic cardiomyopathy; High-glucose; NLRP3; Pyroptosis; UCP2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of First Hospital of Lanzhou University institutional animal care and conducted according to the AAALAC and the IACUC guidelines (LDYYLL 2021 − 307). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Federation ID. IDF Diabetes Atlas. 2025.
Grants and funding
LinkOut - more resources
Full Text Sources

