Relationship between cerebral small vessel disease and proteinopathies in the medial temporal lobe
- PMID: 40671047
- PMCID: PMC12265290
- DOI: 10.1186/s40478-025-02076-y
Relationship between cerebral small vessel disease and proteinopathies in the medial temporal lobe
Abstract
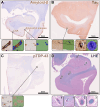
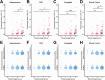
The medial temporal lobe (MTL) is strategically important for cognition and the pathogenesis of Alzheimer's Disease (AD). Cerebral small vessel disease (CSVD) independently contributes to cognitive impairment and is believed to play a role in AD. CSVD and proteinopathies related to AD often coexist in the MTL but neither the severity of CSVD, nor the associations between these pathologies have been quantitatively addressed in this brain region. We hypothesized that the severity of CSVD in the MTL is associated with the local burden of proteinopathies implicated in neurodegeneration (tau-tangles, amyloid-β-plaques, phospho-Tar-DNA-Binding-Protein-43 [pTDP-43]), regardless of disease stage. One potential mechanism linking CSVD and proteinopathies is a failure in perivascular brain clearance. Therefore, the relationship between CSVD and the enlargement of perivascular spaces (PVS) was investigated. AI-models and manual ratings were applied to digitized histological MTL-sections of 152 autopsy cases with and without Alzheimer's Disease Neuropathological Changes to quantify proteinopathies and the two common forms of CSVD, cerebral amyloid angiopathy (CAA) and arteriolosclerosis. The associations between CSVD and proteinopathies were assessed using linear-mixed-effects models. The relationship between CSVD and PVS enlargement was also investigated. Regional CAA-burden increased along Braak-stages and was positively associated with amyloid-β-plaques percentage area and tau-tangles density, irrespective of Braak-stage, but not with density of pTDP-43 inclusions. Local arteriolosclerosis severity was not associated with Braak-stages and had no direct effect on parenchymal proteinopathies. However, arteriolosclerosis severity and its interaction with CAA were positively associated with PVS enlargement. These results suggest that CAA, but not arteriolosclerosis, is more directly implicated in the pathophysiology of proteinopathies in the MTL. Moreover, arteriolosclerosis may contribute to perivascular clearance dysfunction.
Keywords: Alzheimer’s disease (AD); Arteriolosclerosis; Cerebral amyloid angiopathy (CAA); Hippocampus; Medial Temporal lobe (MTL); Perivascular space (PVS).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The MGH institutional review board approved this study. All human participants or their next of kin provided informed consent for brain donation and related research. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D et al (2017) Dementia prevention, intervention, and care. Lancet 390:2673–2734 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

